
Conceptual Physical Science (6th Edition)
6th Edition
ISBN: 9780134060491
Author: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 50TAR
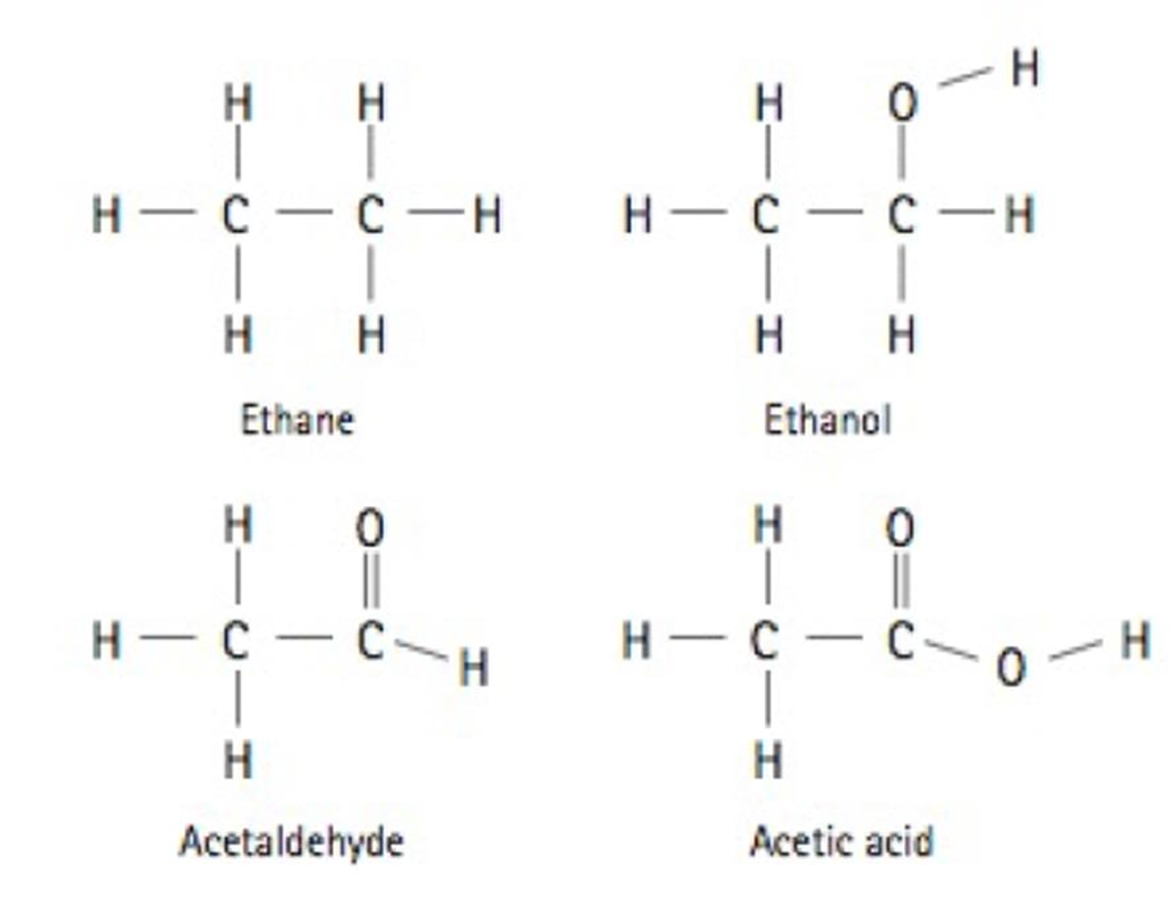
Rank the following molecules from least oxidized to most oxidized:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
suggest a reason ultrasound cleaning is better than cleaning by hand?
Checkpoint 4
The figure shows four orientations of an electric di-
pole in an external electric field. Rank the orienta-
tions according to (a) the magnitude of the torque
on the dipole and (b) the potential energy of the di-
pole, greatest first.
(1)
(2)
E
(4)
What is integrated science.
What is fractional distillation
What is simple distillation
Chapter 18 Solutions
Conceptual Physical Science (6th Edition)
Ch. 18 - What does sulfur dioxide have to do with acid...Ch. 18 - How do humans generate the air pollutant sulfur...Ch. 18 - Prob. 14RCQCh. 18 - Prob. 15RCQCh. 18 - What elements have the greatest tendency to behave...Ch. 18 - Prob. 17RCQCh. 18 - What happens to a reducing agent as it reduces?Ch. 18 - What is electrochemistry?Ch. 18 - What is the purpose of the salt bridge in a...Ch. 18 - Prob. 21RCQ
Ch. 18 - What is the prime difference between a battery and...Ch. 18 - Prob. 23RCQCh. 18 - What is electrolysis, and how does it differ from...Ch. 18 - Prob. 25RCQCh. 18 - Prob. 26RCQCh. 18 - What metal coats a galvanized nail?Ch. 18 - Prob. 28RCQCh. 18 - What is iron forced to accept during cathodic...Ch. 18 - What happens to the polarity of oxygen atoms as...Ch. 18 - Show that the hydroxide ion concentration in an...Ch. 18 - When the hydronium ion concentration of a solution...Ch. 18 - Show that an aqueous solution having a pH of 5 has...Ch. 18 - When the pH of a solution is 1, the concentration...Ch. 18 - Show that the pH of a solution is 0.301 when its...Ch. 18 - Each year about 1.6 107 (16 million) metric tons...Ch. 18 - Prob. 44TASCh. 18 - Prob. 45TARCh. 18 - The three chemicals listed below are all very weak...Ch. 18 - Rank in order of decreasing pH the rain that fell...Ch. 18 - Prob. 48TARCh. 18 - Review the concept of electronegativity in Section...Ch. 18 - Rank the following molecules from least oxidized...Ch. 18 - An acid and a base react to form a salt, which...Ch. 18 - Identify the acid or base behavior of each...Ch. 18 - Prob. 53ECh. 18 - Prob. 54ECh. 18 - The main component of bleach is sodium...Ch. 18 - Prob. 56ECh. 18 - Prob. 57ECh. 18 - Some molecules are able to stabilize a negative...Ch. 18 - Prob. 59ECh. 18 - Within a neutral solution of supercritical water...Ch. 18 - What is the concentration of hydronium ions in a...Ch. 18 - Can an acidic solution be made less acidic by...Ch. 18 - Bubbling carbon dioxide into water causes the pH...Ch. 18 - Pour vinegar onto beach sand from the Caribbean,...Ch. 18 - What happens to the pH of soda water as it loses...Ch. 18 - Prob. 66ECh. 18 - Prob. 67ECh. 18 - Prob. 68ECh. 18 - Prob. 69ECh. 18 - Hydrogen sulfide, H2S, burns in the presence of...Ch. 18 - Unsaturated fatty acids, such as C12H22O2, react...Ch. 18 - The type of iron that the human body needs for...Ch. 18 - Chemical equations need to be balanced not only in...Ch. 18 - Prob. 74ECh. 18 - Why does a salt bridge last only so long?Ch. 18 - How does turning on the radio while you are...Ch. 18 - What are some key advantages that a fuel-cell...Ch. 18 - Why would a miniaturized fuel cell require a...Ch. 18 - Prob. 79ECh. 18 - Prob. 80ECh. 18 - Copper atoms have a greater tendency to be reduced...Ch. 18 - Clorox is a laundry bleaching agent used to remove...Ch. 18 - Pennies manufactured after 1982 are made of zinc...Ch. 18 - Prob. 84ECh. 18 - Prob. 85ECh. 18 - Water is 88.88% oxygen by mass. Oxygen is exactly...Ch. 18 - Why is the air over an open flame always moist?Ch. 18 - Upon ingestion, grain alcohol, C2H6O, is...Ch. 18 - Your body creates chemical energy from the food...Ch. 18 - Do the digestion and subsequent metabolism of...Ch. 18 - Why is it easier for the body to excrete a polar...Ch. 18 - What is the relationship between the hydroxide ion...Ch. 18 - Prob. 2RATCh. 18 - Sodium hydroxide, NaOH, is a strong base, which...Ch. 18 - Prob. 4RATCh. 18 - Why do we use the pH scale to indicate the acidity...Ch. 18 - When the hydronium ion concentration equals 1...Ch. 18 - Prob. 7RATCh. 18 - Prob. 8RATCh. 18 - How does an atum's electronegativity relate to its...Ch. 18 - Why does a battery that has thick zinc walls last...
Additional Science Textbook Solutions
Find more solutions based on key concepts
5. The diploid number of the hypothetical animal Geneticus introductus is 2n = 36. Each diploid nucleus contain...
Genetic Analysis: An Integrated Approach (3rd Edition)
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology with Physiology (5th Edition)
Two culture media were inoculated with four different bacteria. After incubation, the following results were ob...
Microbiology: An Introduction
27. Consider the reaction.
Express the rate of the reaction in terms of the change in concentration of each of...
Chemistry: Structure and Properties (2nd Edition)
Where is transitional epithelium found and what is its importance at those sites?
Anatomy & Physiology (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 19:39 · C Chegg 1 69% ✓ The compound beam is fixed at Ę and supported by rollers at A and B. There are pins at C and D. Take F=1700 lb. (Figure 1) Figure 800 lb ||-5- F 600 lb بتا D E C BO 10 ft 5 ft 4 ft-—— 6 ft — 5 ft- Solved Part A The compound beam is fixed at E and... Hình ảnh có thể có bản quyền. Tìm hiểu thêm Problem A-12 % Chia sẻ kip 800 lb Truy cập ) D Lưu of C 600 lb |-sa+ 10ft 5ft 4ft6ft D E 5 ft- Trying Cheaa Những kết quả này có hữu ích không? There are pins at C and D To F-1200 Egue!) Chegg Solved The compound b... Có Không ☑ ||| Chegg 10 וחarrow_forwardNo chatgpt pls will upvotearrow_forwardNo chatgpt pls will upvotearrow_forward
- No chatgpt pls will upvotearrow_forwardair is pushed steadily though a forced air pipe at a steady speed of 4.0 m/s. the pipe measures 56 cm by 22 cm. how fast will air move though a narrower portion of the pipe that is also rectangular and measures 32 cm by 22 cmarrow_forwardNo chatgpt pls will upvotearrow_forward
- 13.87 ... Interplanetary Navigation. The most efficient way to send a spacecraft from the earth to another planet is by using a Hohmann transfer orbit (Fig. P13.87). If the orbits of the departure and destination planets are circular, the Hohmann transfer orbit is an elliptical orbit whose perihelion and aphelion are tangent to the orbits of the two planets. The rockets are fired briefly at the depar- ture planet to put the spacecraft into the transfer orbit; the spacecraft then coasts until it reaches the destination planet. The rockets are then fired again to put the spacecraft into the same orbit about the sun as the destination planet. (a) For a flight from earth to Mars, in what direction must the rockets be fired at the earth and at Mars: in the direction of motion, or opposite the direction of motion? What about for a flight from Mars to the earth? (b) How long does a one- way trip from the the earth to Mars take, between the firings of the rockets? (c) To reach Mars from the…arrow_forwardNo chatgpt pls will upvotearrow_forwarda cubic foot of argon at 20 degrees celsius is isentropically compressed from 1 atm to 425 KPa. What is the new temperature and density?arrow_forward
- Calculate the variance of the calculated accelerations. The free fall height was 1753 mm. The measured release and catch times were: 222.22 800.00 61.11 641.67 0.00 588.89 11.11 588.89 8.33 588.89 11.11 588.89 5.56 586.11 2.78 583.33 Give in the answer window the calculated repeated experiment variance in m/s2.arrow_forwardNo chatgpt pls will upvotearrow_forwardCan you help me solve the questions pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY