
Draw the structure for each of the following:
a. phenol
b. benzyl phenyl ether
c. benzonitrile
d. benzaldehyde
e. anisole
f. styrene
g. toluene
h. tert-buty lbenzene
i. benzyl chloride
a)
Interpretation:
The structure of phenol is to be drawn.
Concept introduction:
Phenols are defined as those compounds in which hydroxy group is attached directly to the benzene ring. Phenols and alcohols have so many similar properties.
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of phenol is shown below.
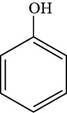
Figure 1
Explanation of Solution
The structure of phenol is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of phenol is shown below.
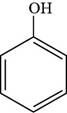
Figure 1
b)
Interpretation:
The structure of benzyl phenyl ether is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzyl phenyl ether is shown below.
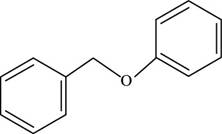
Figure 2
Explanation of Solution
The structure of benzyl phenyl ether is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzyl phenyl ether is shown below.

Figure 2
c)
Interpretation:
The structure of benzonitrile is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzonitrile is shown below.
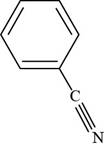
Figure 3
Explanation of Solution
The structure of benzonitrile is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzonitrile is shown below.
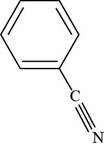
Figure 3
d)
Interpretation:
The structure of benzaldehyde is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzaldehyde is shown below.
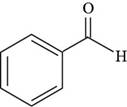
Figure 4
Explanation of Solution
The structure of benzaldehyde is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzaldehyde is shown below.
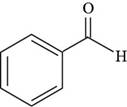
Figure 4
e)
Interpretation:
The structure of anisole is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of anisole is shown below.
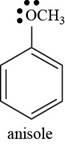
Figure 5
Explanation of Solution
The structure of anisole is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of anisole is shown below.
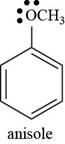
Figure 5
f)
Interpretation:
The structure of styrene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of styrene is shown below.
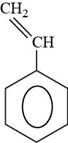
Figure 6
Explanation of Solution
The structure of styrene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of styrene is shown below.
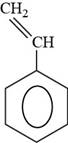
Figure 6
g)
Interpretation: The structure of toluene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of toluene is shown below.
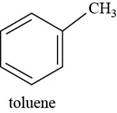
Figure 7
Explanation of Solution
The structure of toluene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of toluene is shown below.
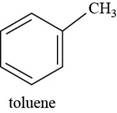
Figure 7
h)
Interpretation: The structure of tert-butyl benzene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of tert-butyl benzene is shown below.
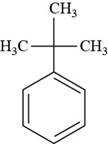
Figure 8
Explanation of Solution
The structure of tert-butyl benzene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of tert-butyl benzene is shown below.

Figure 8
i)
Interpretation:
The structure of benzyl chloride is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzyl chloride is shown below.
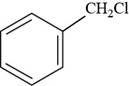
Figure 9
Explanation of Solution
The structure of benzyl chloride is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzyl chloride is shown below.

Figure 9
Want to see more full solutions like this?
Chapter 18 Solutions
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
- 10. Draw a structural formula for each of the fol 'owing esters: a. cyclohexyl propanoate b. methyl formate c. ethyl benzoate d. isopropyl acetate e. butyl butanoate f. propyl pentanoatearrow_forwardPhenol is Select one: a. CH3CH2CH2OH b. C8H11OH c. CH3CH2CH2CH2CH2OH d. C6H5OHarrow_forwardMestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills. Select all of the functional group families, to which mestranol belongs. Choose one or more: A. Ester B. Alkene C. Ether D. Thiol E. Alcohol F. Arene G.Aldehyde H.Ketone I. Alkynearrow_forward
- List the following compounds in order of increasing water solubility: a. hexane c. 3-pentanone b. 2-pentanol d. pentanoic acidarrow_forwardDraw the structure for each of the following: a. isopropyl alcohol b. isopentyl fluoride c. sec-butyl iodide d. tert-pentyl alcohol e. tert-butylamine f. n-octyl bromidearrow_forward1. Chief organic component of vinegar a. acetic acid b. formic acid c. benzoic acid d. butanoic acid 2. This term means without water. a. carbonyl b. hydroxyl c. anhydride d. carboyl 3. Compounds containing the cyano group. a. nitriles b. amides c. amines d. nitrates 4. General formula of a Grignard reagent. a. RCOX b. RCN c. RCOOH d. RMgX 5. Organic derivatives of ammonia, derived from replacing one, two or all three hydrogens of the ammonia. a. amide b. amine c. cyan d. nitro 6. Sulfur analogs of alcohols where the O in R-OH is replaced by sulfur. a. Thioesters b. Thiols c. Thioaldehydes d. Thioethers 7. General formula of alkanes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 8. General formula of alkenes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 9. General formula of alkynes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 10. Which is soluble in water? a. methanol b. ethanol c. propanol d. all of the above 11. Which substance will have the highest boiling point? a. methanol b.…arrow_forward
- 13. The ferric chloride (FeCb) solution is used as a test for: a. Alcohols d. Alkyl halides b. Phenols c. Carboxylic Acids e. Alkenesarrow_forwardDraw each of the following compounds, using condensed formulas: a. p-Xylene b. Isopropylbenzene c. m-Nitroanisole d. p-Methylbenzaldehydearrow_forwardEsterfication is the reaction of carboxylic acid with Select one: a. water b. alcohol c. alkyl halide d. ammoniaarrow_forward
- What kind of solvent ingredients is usually used in the concentrations of 4-10 percent in skin care products and their function is to soften skin cells and to lessen wrinkles? A. Ethly acetate B. Alpha hydroxyl acids C. Phenols and phenol derivatives D. Aliphatic alcoholsarrow_forwardDraw condensed formulas for each of the following compounds:a. Methyl benzoateb. Butyl decanoatec. Methyl propionated. Ethyl propionatearrow_forwardDraw the structures of the following compound and give it's IUPAC name: a. Methyl mercaptan b. sec-BUtyl mercaptan c. 2-Methyl-2-propanethiol d. 1-Pentanethiolarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





