
Interpretation: The given following transformations should be determined.
Concept Introduction:
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of
Electrophilic substitution reactions are
Answer to Problem 38PP
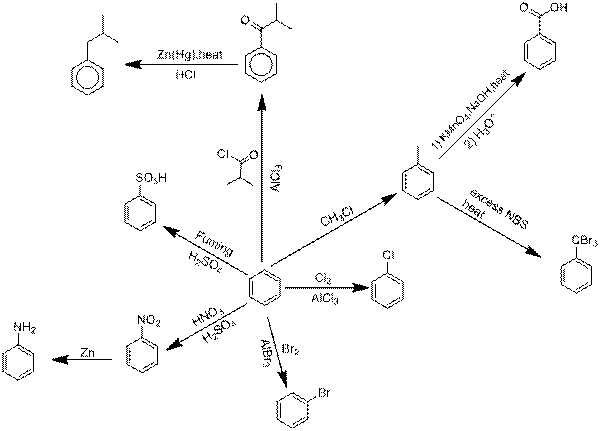
Explanation of Solution
Chlorination of benzene.
Benzene reacts with Chlorine in an electrophilic substitution reaction, other than only in the presence of a catalyst. These compounds act as the catalyst and perform precisely like Aluminium chloride in these reactions.
Bromination of benzene.
Explanation: Benzene reacts with Bromine in an electrophilic substitution reaction, other than only in the presence of a catalyst. These compounds act as the catalyst and perform precisely like Aluminium bromide in these reactions.
Nitration followed by nitro reduction.
Nitration is a universal group of chemical procedure for the opening of a nitro group keen on an organic chemical compound. Extra loosely the term as well is applied imperfectly to the different process of forming nitrate esters between alcohols and nitric acid. Nitro group is converted into
Sulfonation of benzene.
Sulfonation is a reversible reaction so as to produces Benzene sulfonic acid by adding fuming Sulfuric acid.
Friedel-Crafts acyation followed by Clemmensen reduction.
Friedel-Crafts acyation of benzene linking an electrophilic substitution reaction between benzene and alkyl chloride in the presence of an Aluminium chloride catalyst.
Clemmensen reduction is a chemical reaction explain because a reduction of
Friedel Crafts alkylation followed by Oxidation.
Friedel Crafts alkylation involves the alkylation of an
The given following transformations should be determined.
Want to see more full solutions like this?
Chapter 18 Solutions
ORG.CHEM.WILEYPLUSNEXTGEN.W/LLTEXT+STDY.
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





