
Physics for Scientists and Engineers
6th Edition
ISBN: 9781429281843
Author: Tipler
Publisher: MAC HIGHER
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 17, Problem 35P
(a)
To determine
The graph of length versus temperature for mercury.
Given:
The length of column of mercury in the thermometer is 4 cm
The pressure of water is 1 atm
The length of column of mercury in the thermometer is 4 cm
The thermometer in the boiling water is 24 cm
Introduction:
Explanation:
The graph of length of the mercury column versus temperature is:
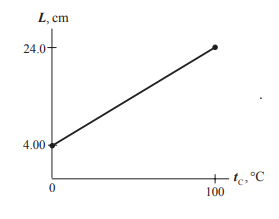
The equation for the graph is:
L = m T + C
Here, L cm T C
Substitute 0.200 cm / °C m 4.0 cm C
L = ( 0.200 cm / °C ) T + 4.0 cm
Conclusion:
The graph of length versus temperature for mercury.
Given:
The length of column of mercury in the thermometer is
The pressure of water is
The length of column of mercury in the thermometer is
The thermometer in the boiling water is
Introduction:
Explanation:
The graph of length of the mercury column versus temperature is:

The equation for the graph is:
Here,
Substitute
Conclusion:
(b)
To determine
The length of the column when the temperature is
(b)
To determine
The temperature when the length of the mercury column is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1.7. The length of the mercury column in the old-fashioned mercury-in-glass thermometer
is 15.00 cm when the thermometer is in contact with water at its triple point. Consider
the length of the mercury column as the thermometric property X and let 0 be the
empirical temperature determined by this thermometer.
(a) Calculate the empirical temperature when the length of the mercury column is
275.16411
32°f
Q ax
-37.40142F
19.00 cm.
(b) If X can be measured with a precision of 0.01 cm, can this thermometer distin-
guish between the normal freezing point of water and the triple point of water?
611+23F
23)
A balloon is filled with helium gas at atmospheric pressure (1 atm) until its volume is 800
m³. The helium gas is then transferred to cylinders that have a volume of 2.3 m³ at a pres-
sure of 13.3 atm.
Calculate the number of cylinders used. Assume that the temperature of the helium gas
remains constant. (1 atm = 1.013 × 105 Pa).
A Fahrenheit and a Celsius thermometer are both immersed in a fluid. a) If the two numerical readingsare identical, what is the temperature of the fluid expressed in ºK and ºR. b) If the Fahrenheittemperature is numerically twice that of the Celsius reading, what is the temperature of the fluidexpressed in ºK and ºR?
Chapter 17 Solutions
Physics for Scientists and Engineers
Ch. 17 - Prob. 1PCh. 17 - Prob. 2PCh. 17 - Prob. 3PCh. 17 - Prob. 4PCh. 17 - Prob. 5PCh. 17 - Prob. 6PCh. 17 - Prob. 7PCh. 17 - Prob. 8PCh. 17 - Prob. 9PCh. 17 - Prob. 10P
Ch. 17 - Prob. 11PCh. 17 - Prob. 12PCh. 17 - Prob. 13PCh. 17 - Prob. 14PCh. 17 - Prob. 15PCh. 17 - Prob. 16PCh. 17 - Prob. 17PCh. 17 - Prob. 18PCh. 17 - Prob. 19PCh. 17 - Prob. 20PCh. 17 - Prob. 21PCh. 17 - Prob. 22PCh. 17 - Prob. 23PCh. 17 - Prob. 24PCh. 17 - Prob. 25PCh. 17 - Prob. 26PCh. 17 - Prob. 27PCh. 17 - Prob. 28PCh. 17 - Prob. 29PCh. 17 - Prob. 30PCh. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Prob. 33PCh. 17 - Prob. 34PCh. 17 - Prob. 35PCh. 17 - Prob. 36PCh. 17 - Prob. 37PCh. 17 - Prob. 38PCh. 17 - Prob. 39PCh. 17 - Prob. 40PCh. 17 - Prob. 41PCh. 17 - Prob. 42PCh. 17 - Prob. 43PCh. 17 - Prob. 44PCh. 17 - Prob. 45PCh. 17 - Prob. 46PCh. 17 - Prob. 47PCh. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - Prob. 51PCh. 17 - Prob. 52PCh. 17 - Prob. 53PCh. 17 - Prob. 54PCh. 17 - Prob. 55PCh. 17 - Prob. 56PCh. 17 - Prob. 57PCh. 17 - Prob. 58PCh. 17 - Prob. 59PCh. 17 - Prob. 60PCh. 17 - Prob. 61PCh. 17 - Prob. 62PCh. 17 - Prob. 63PCh. 17 - Prob. 64PCh. 17 - Prob. 65PCh. 17 - Prob. 66PCh. 17 - Prob. 67PCh. 17 - Prob. 68PCh. 17 - Prob. 69PCh. 17 - Prob. 70PCh. 17 - Prob. 71PCh. 17 - Prob. 72PCh. 17 - Prob. 73PCh. 17 - Prob. 74PCh. 17 - Prob. 75PCh. 17 - Prob. 76PCh. 17 - Prob. 77PCh. 17 - Prob. 78PCh. 17 - Prob. 79PCh. 17 - Prob. 80P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- At 25.0 m below the surface of the sea, where the temperature is 5.00C, a diver exhales an air bubble having a volume of 1.00 cm3. If the surface temperature of the sea is 20.0C, what is the volume of the bubble just before it breaks the surface?arrow_forwardAssuming the human body is primarily made of water, estimate the number of molecules in it. (Note that water has a molecular mass of 18 g/mol and there are roughly 1024 atoms in a mole)arrow_forwardIf a 4 m3 of gas initially at STP is placed under a pressure of 3 atm, the temperature of the gas rises to 27◦C. What is the volume now? Calculate to 2 decimals.arrow_forward
- The pressure P (in kilopascals), volume V (in liters), and temperature T (in kelvins) of a mole of an ideal gas are related by the equation PV = 8.317. Find the rate at which the volume is changing when the temperature is 325 K and increasing at a rate of 0.05 K/s and the pressure is 29 and increasing at a rate of 0.07 kPa/s. Please show your answers to at least 4 decimal places. dV dt L/sarrow_forwardThe second pic is the rquired number of tolerance needed in the answerarrow_forwardA gas is heated to 75 °C and a pressure of 200 kPa. If the container is compressed to hold a volume of 700 mL, what was the volume of the gas, (in litres), at a temperature of 40 °C and 100 kPa pressure? B. A vessel of volume 5x10-5 m3 contains hydrogen at a pressure of 1.5 Pa at a temperature of 37 °C. Estimate: i. The number of molecules of hydrogen gas in the vessel. (4 marks) ii. The number of moles of hydrogen. iii. Mass of hydrogen iv. The kinetic energy of hydrogen. v. The root mean square speed. Given: M = 1.008 g/mol; R = 8.31 J/mol-K; NA = 6.02 x 1023 mol-1; k = 1.38 x 10-23 J/Karrow_forward
- A diver is at 5 meter depth in a fresh water lake. At that depth, the temperature is 15◦C. The pressure in the water at a depth d is given by p(d) = psurface + ρgd, where ρ is the liquid density, and g = 9.81m s−2is the gravitational constant.(a) The diver releases an air bubble of 1cm diameter. What is the diameter of the bubble when it reaches the surface where the temperature is 20◦C?Assume that the bubble temperature is always the same as the surroundingwater.(b) The diver stayed 1h at a 5m depth. The pressure in the diving tank went down from 250 bars to 50 bars. We assume that the volume of the lungs is 5L. Assuming that the water temperature is also 15◦C, how long could the diver have stayed underwater at 20m depth using the same amount of air?(c) The diver is at 5m depth and decides to surface. The air in the lungs is at a constant temperature of 37◦C. What fraction of the air in her/his lungs should the diver inhale/release to maintain a constant lung volume?arrow_forwardA (1.0x10^1) liter bottle is filled with nitrogen (N2) at STP (Standard Temperature and Pressure is 1 atm and 273 K) and closed tight. If the temperature is raised to 100° C, what will be the new pressure in SI units to two significant figures.arrow_forwardWhen air expands adiabatically (without gaining or losing heat), its pressure P and volume V are related by the equation PV14 = Cwhere C is a constant. Suppose that at a certain instant the volume is 670 cubic centimeters and the pressure is 99 kPa and is decreasing at a rate of 7 kPa/minute. At what rate in cubic centimeters per minute is the volume increasing at this instant? cm3 min (Pa stands for Pascal -- it is equivalent to one Newton/(meter squared); kPa is a kiloPascal or 1000 Pascals. )arrow_forward
- Either give an exact answer, or make sure you include at least 4 significant digits on your answer. The pressure P (in kilopascals), volume V (in liters), and temperature T (in kelvins) of a mole of an ideal gas are related by the equation PV = 8.31T. Find the rate at which the volume is changing when the temperature is 330 K and increasing at a rate of 0.15 K/s and the pressure is 26 and increasing at a rate of 0.03 kPa/s. 1110 L/Sarrow_forwardWhat are the gallons consumed in units of gallons per winter?arrow_forwardA balloon is filled with helium at a pressure of 2.4 * 105Pa. The balloon is at a temperature of 18 °Cand has a radius of 0.25 m. (a) How many helium atoms arecontained in the balloon? (b) Suppose we double the numberof helium atoms in the balloon, keeping the pressure and thetemperature fixed. By what factor does the radius of the balloonincrease? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Heat Transfer: Crash Course Engineering #14; Author: CrashCourse;https://www.youtube.com/watch?v=YK7G6l_K6sA;License: Standard YouTube License, CC-BY