
What would be the effect of carrying out the sodium iodide in acetone reaction with the
Interpretation: The effect of carrying out the sodium iodide in acetone reaction with the alkyl halide using iodide solution half as concentrated is to be interpreted.
Concept introduction: The reaction of alkyl halide with sodium iodide results the formation of alkyl iodide. The reaction occurs in the presence of acetone as the solvent. It is named as Finkelstein reaction and is followed by
Answer to Problem 1Q
In the presence of half concentrated iodide solution, the
Explanation of Solution
The mechanism of the given reaction can be shown as given below:
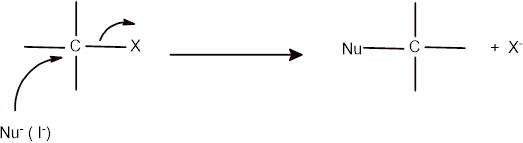
Here the iodide ions act as nucleophile and involve in the rate determining step as reaction follows the
Therefore, with decrease in the concentration of iodide ion, the rate of reaction will also halves that yield only half of the product.
In
Want to see more full solutions like this?
Chapter 17 Solutions
EBK MACROSCALE AND MICROSCALE ORGANIC E
- If you carried out the sodium iodide-in-acetone reactions on the alkyl halides using an iodide solution that was half as concentrated, what would be the effect on reaction rate? In addition to noting whether the reaction rate would be faster or slower, you must fully justify your answer using a DETAILED explanation that demonstrates your understanding of the kinetics of nucleophilic substitution. No credit will be given for vague answers.arrow_forwardGive the reaction mechanism for ETHYNE and bromine solution. Draw and explain the steps of the reaction mechanism.arrow_forwardDraw the structure of benzoic acid and explain which product will be formed as a result of the reaction with the nitronium ion in the electrophilic aromatic displacement reaction.arrow_forward
- Which reacts fastest & slowest with m-chloroperbenzoic acid, why?arrow_forwardA common method where one alkyl halide is converted to another alkyl halide is known as Finkelstein reaction. In one example of this reaction the reaction of an alkyl chloride with potassium iodide is generally carry out in acetone to maximize the amount of alkyl iodine that this formed. Why does the solvent increase the amount of alkyl iodide. Hint: potassium idioide is soluble in acetone but potassium chloride is not.arrow_forwardIn the Iodination of Acetone, What will happen to the reaction time and reaction rate if the concentration of one of the reactants is doubled while keeping everything the same?arrow_forward
- Rationalize the selectivity observed in the benzil reduction. Draw the transition states of the nucleophilic attack leading to either meso or racemic products and indicate why one is more stable than the other.arrow_forwardExplain why attempts to use esters for Friedel-Crafts acylation always lead to a complex mixture of alkylated and acylated products.arrow_forwardWhich one undergoes the fastest reaction with methanol in acidic condition?arrow_forward
- Carboxylic acids are lowest in reactivity towards nucleophilic substitution. Why? (Consider the reaction between acetic acid and sodium methoxide).arrow_forwardExplain using resonance structures of the intermediates (please explain and draw them out) why the bromination of phenol is faster than the bromination of phenyl ester?arrow_forwardIf the melting point obtained was 144 degrees Celsius, which structure corresponds to the mixed aldol product obtained.arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

