
Concept explainers
(a)
Interpretation:
The ester formed on treating 2-propanol with CH3CH2COOH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.68P
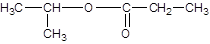
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2COOH (propanoic acid) and 2-propanol is:
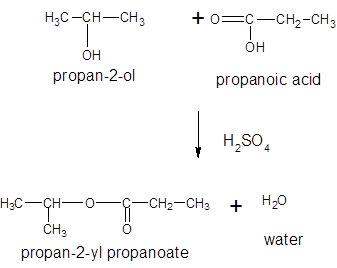
(b)
Interpretation:
The ester formed on treating 2-propanol with following in the presence of H2SO4 should be determined:
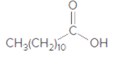
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.68P
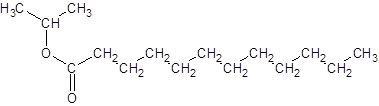
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3(CH2)10COOH (dodoecanoic acid) and 2-propanol is:
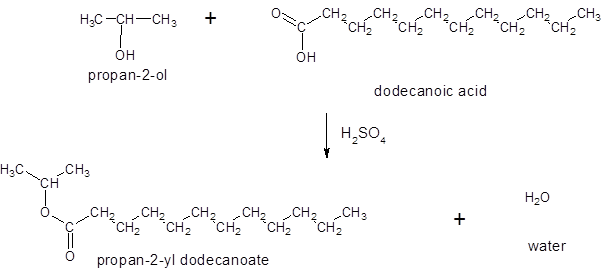
(c)
Interpretation:
The ester formed on treating 2-propanol with HCO2H in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.68P
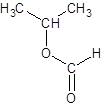
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between HCO2H (formic acid) and 2-propanol is:
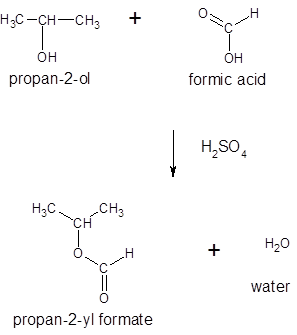
(d)
Interpretation:
The ester formed on treating 2-propanol with following in the presence of H2SO4 should be determined:
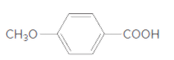
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.68P
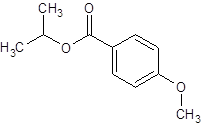
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between 4-methoxybenzoic acid and 2-propanol is:
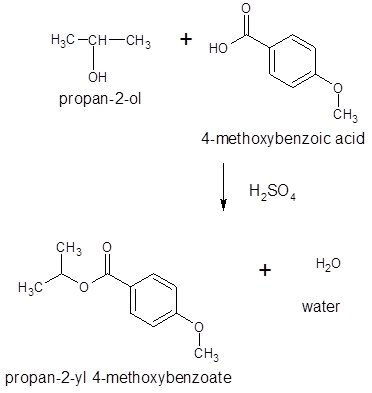
Want to see more full solutions like this?
Chapter 17 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Classify each alkyl halide as 1°, 2°, or 3°. CH3 c. CHg-C-CHCH3 ČH3 ČI CH;CH2CH,CH,CH2-Br b. d. a.arrow_forwardWhich is a thiol? CH3 HS SH b. HO. CH3 H.CS CHa CH C. H. d. భిగింగడి 工 a.arrow_forwardOH 15. CH3-C-H ÓCH, The compound above is an example of a(n) a. acetal b. ketal O c. hemiacetal d. hemiketal e. none of the abovearrow_forward
- Show how to bring about each conversion in good yield. a. b. C6H5 Cl OH COOH C6H5 COOHarrow_forward7 8 11 12 me CH3 H3C 3-butylpropanoate 2-butoxypropane propyl-2-butanoate 2-butylpropanoate H₂ C CH3arrow_forward8 What ester is formed when each carboxylic acid is treated with 2-propanol [(CH 3) 2CHOH] in the presence of H 2SO 4?arrow_forward
- 18. Ketone reduction Dicyclohexyl ketone Reduce the ketone. 1. NaBH4, ethanol 2. H3O+ H OH Dicyclohexylmethanol (88%) (a 2° alcohol)arrow_forwardWhat is the preferred product in the following reaction? H.C Br NaOH the alkene The alcohol H₂C OH + H₂C CH₂arrow_forwardDraw out each compound to clearly show what groups are bonded to the carbonyl carbon. Label each compound as a ketone or aldehyde. a. CH 3CH 2CHO b. CH 3CH 2COCH 3 c. (CH 3) 3CCOCH 3 d. (CH 3CH 2) 2CHCHOarrow_forward
- Does the equilibrium favor the reactants or products in each substitution reaction? a. CH;CH2-NH2 Br CH;CH2-Br + "NH2 b. "CN CN + I-arrow_forwardDetermine the products of each carboxylic acid reaction. a. CH3-CH -C-OH + NaOH b. CH;-CH2-CH;-C-OH + CH — CH, —Сн, — онarrow_forwardWhat is the reduction product of the following compound with H2/Pd? A 2-propyl-1-cyclohexanol B 2-propenyl-1-cyclohexanol C2-propylcyclohexanone D 1-propyl-2-cyclohexanol What is the oxidation product of this compound? H' A ethanoic acid acetic acid propanoic acid D propanone What is the oxidation product of this compound? A 3,4-dimethylpentanoic acid B 2,3-dimethylpentanoic acid C2,3-dimethylpentanone 3,4-dimethylpentanonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





