
Concept explainers
What ester is formed when butanoic acid
a.
b.
c.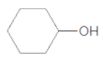
d.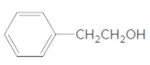
(a)
Interpretation:
The ester formed on treating butanoic acid with CH3OH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.67P
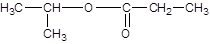
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and methanol is:
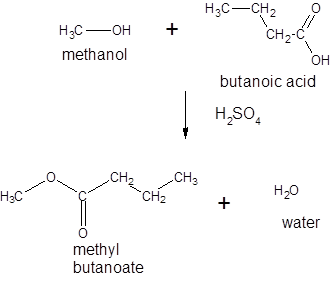
(b)
Interpretation:
The ester formed on treating butanoic acid with CH3CH2CH2OH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.67P
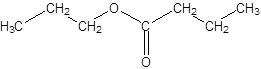
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and propanol is:
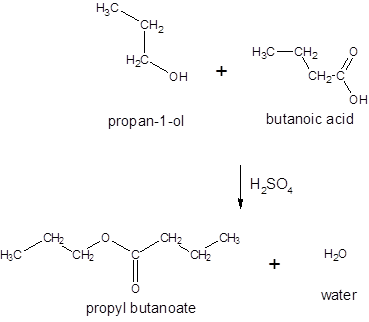
(c)
Interpretation:
The ester formed on treating butanoic acid with following alcohol in the presence of H2SO4 should be determined:
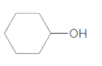
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.67P
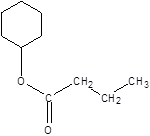
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and cyclohexanol is:
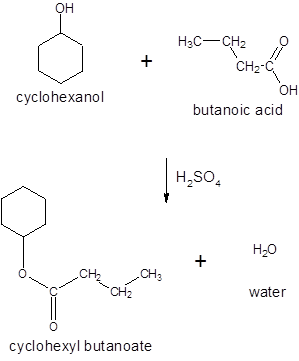
(d)
Interpretation:
The ester formed on treating butanoic acid with following alcohol in the presence of H2SO4 should be determined:
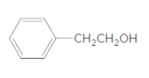
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.67P
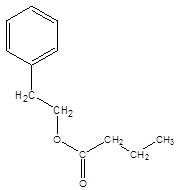
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and 2-phenylethan-1-ol is:
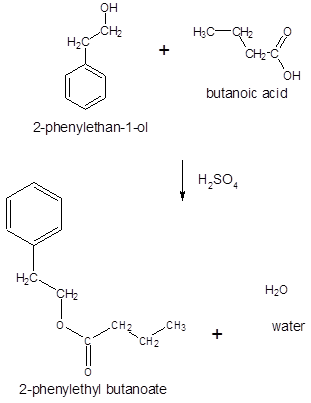
Want to see more full solutions like this?
Chapter 17 Solutions
General, Organic, & Biological Chemistry
- MCQ 3: The carbonyl group in aldehyde is A. C=O B. C-O C. CO D. CHOarrow_forwardWhat is the preferred product in the following reaction? H.C Br NaOH the alkene The alcohol H₂C OH + H₂C CH₂arrow_forward• Whał arc the IUPAC namar of the ff. Carboxylic acias? a. COOH COOH CH3 b. I f. .COOH CHJCH2 ÇHCHCOOH CH3 NO2 tON C. COOH ноос d. COOHarrow_forward
- Classify each alkyl halide as 1°, 2°, or 3°. CH3 c. CHg-C-CHCH3 ČH3 ČI CH;CH2CH,CH,CH2-Br b. d. a.arrow_forwardWhat alkenes are formed when each alcohol is dehydrated with TSOH? Label the major product when a mixture results. OH Xom OH a. OH b. CH₂CH3 OH C. d. CH₂CH₂CH₂CH₂OH e.arrow_forwardList the products of each alcohol reaction. CH3 a. CH,-C-OH CH, NazCrO b. CH3-CH-CH;-CH2-OH c. CH-CH-OH +HCI -arrow_forward
- Dehydration of alcohols can be done by using: O Fe2O3 O Cao O all of these O conc. H2SO4arrow_forward1. Name the following ether. 2. Name the following thiol. C C-C-C-C-0-C-C -9-9-⁹ C-C-C-C-C-S-H C-C-C-C 3. Will the ether H bond with water? Will the thiol H bond with water?arrow_forwardShow how to bring about each conversion in good yield. a. b. C6H5 Cl OH COOH C6H5 COOHarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
