
Concept explainers
(a)
Interpretation:
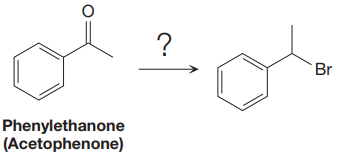
The synthesis of given compound from
Concept introduction:
In an
Answer to Problem 17.66P
The synthesis of given compound from
Explanation of Solution
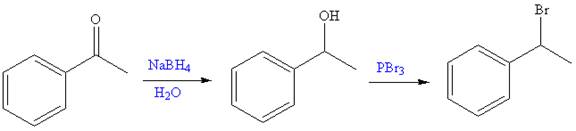
The given reaction is,
The first step of the reaction is reduction of
The synthesis of given compound from
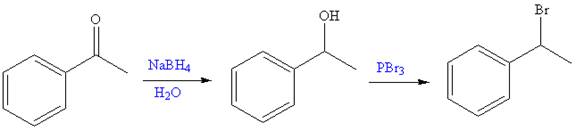
The synthesis of given compound from
(b)
Interpretation:
The synthesis of given compound from
Concept introduction:
The
Answer to Problem 17.66P
The synthesis of given compound from
Explanation of Solution
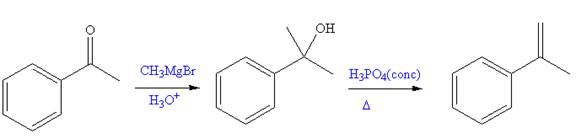
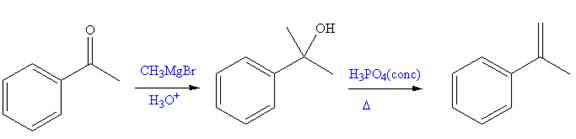
The given reaction is,
In the first a new C-C bond is formed and a methyl group is added to the carbonyl carbon. Both alkyllithium reagents and Grignard reagents can react rapidly with water in a substantially exothermic proton transfer reaction to produce an alkane and
The synthesis of given compound from
The synthesis of given compound from
(c)
Interpretation:
The synthesis of given compound from
Concept introduction:
In an
Answer to Problem 17.66P
The synthesis of given compound from
Explanation of Solution
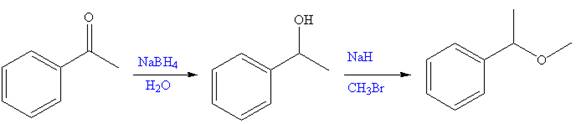
The given reaction is,
The first step of the reaction is reduction of
The synthesis of given compound from
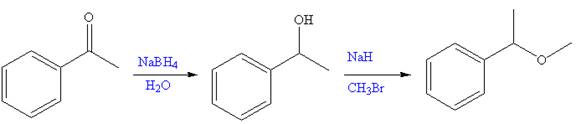
The synthesis of given compound from
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Ph heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
