
Concept explainers
(a)
Interpretation: The structure corresponding to given IUPAC name of a compound is to be drawn.
Concept introduction:
1. When the ring is mono-substituted, then there is no need of numbering.
2. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
3. When the ring is substituted with different substituents, then the numbering is done on the basis of priority.
4. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.4P
The structure corresponding to given compound is,
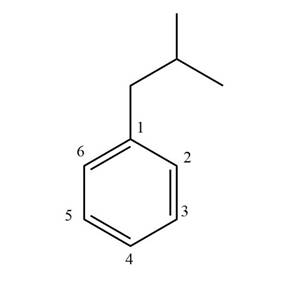
Figure 1
Explanation of Solution
The IUPAC name of the compound is isobutyl benzene. The parent ring is benzene.
Figure 1
The structure corresponding to given compound is shown in Figure 1.
(b)
Interpretation: The structure corresponding to given IUPAC name of a compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. When the ring is mono-substituted, then there is no need of numbering.
2. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
3. When the ring is substituted with different substituents, then the numbering is done on the basis of priority.
4. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.4P
The structure corresponding to given compound is,
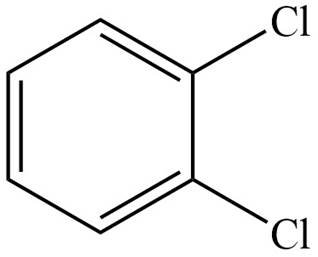
Figure 2
Explanation of Solution
The IUPAC name of the compound is

Figure 2
The structure corresponding to given compound is shown in Figure 2.
(c)
Interpretation: The structure corresponding to given IUPAC name of a compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. When the ring is mono-substituted, then there is no need of numbering.
2. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
3. When the ring is substituted with different substituents, then the numbering is done on the basis of priority.
4. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.4P
The structure corresponding to given compound is,
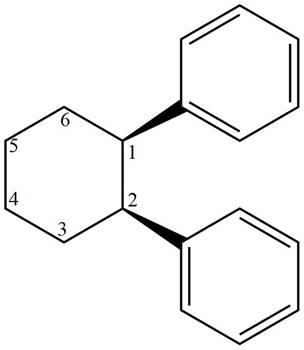
Figure 3
Explanation of Solution
The IUPAC name of the compound is

Figure 3
The structure corresponding to given compound is shown in Figure 3.
(d)
Interpretation: The structure corresponding to given IUPAC name of a compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. When the ring is mono-substituted, then there is no need of numbering.
2. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
3. When the ring is substituted with different substituents, then the numbering is done on the basis of priority.
4. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.4P
The structure corresponding to given compound is,
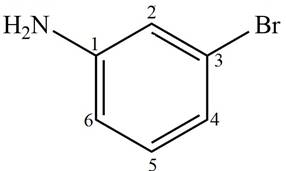
Figure 4
Explanation of Solution
The IUPAC name of the compound is

Figure 4
The structure corresponding to given compound is shown in Figure 4.
(e)
Interpretation: The structure corresponding to given IUPAC name of a compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. When the ring is mono-substituted, then there is no need of numbering.
2. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
3. When the ring is substituted with different substituents, then the numbering is done on the basis of priority.
4. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.4P
The structure corresponding to given compound is,
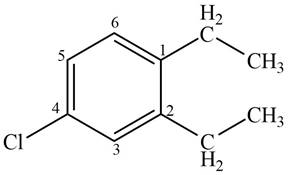
Figure 5
Explanation of Solution
The IUPAC name of the compound is

Figure 5
The structure corresponding to given compound is shown in Figure 5.
(f)
Interpretation: The structure corresponding to given IUPAC name of a compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. When the ring is mono-substituted, then there is no need of numbering.
2. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
3. When the ring is substituted with different substituents, then the numbering is done on the basis of priority.
4. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.4P
The structure corresponding to given compound is,
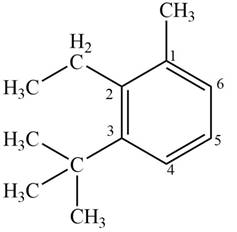
Figure 6
Explanation of Solution
The IUPAC name of the compound is
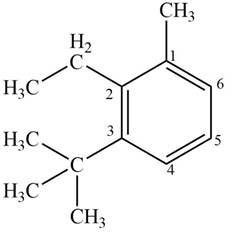
Figure 6
The structure corresponding to given compound is shown in Figure 6.
Want to see more full solutions like this?
Chapter 17 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Draw the expanded formula of the following compounds: (a) 3,3-Diethyl-2,5-dimethylnonane (b) 3-Cyclobutylhexane (c) 1,3-Dibromo-5-methylcyclohexanearrow_forwardDraw a structure for each compound (includes old and new names).(a) 3-methylpent-1-ene (b) cis-3-methyl-3-hexene (c) 3,4-dibromobut-1-ene(d) 1,3-cyclohexadienearrow_forwardDraw the structure that corresponds with each name.(a) 3-ethyloctane (b) 4-isopropyldecane (c) sec-butylcycloheptane(d) 2,3-dimethyl-4-propylnonane (e) 2,2,4,4-tetramethylhexane (f) trans-1,3-diethylcyclopentane(g) cis-1-ethyl-4-methylcyclohexane (h) isobutylcyclopentane (i) tert-butylcyclohexane(j) pentylcyclohexane (k) cyclobutylcyclohexane (l) cis-1-bromo-3-chlorocyclohexanearrow_forward
- 3) Give the IUPAC names for the following structures: (a) NH2 NO2 (c) (d) C-H NH2 Br H3Carrow_forwardDraw the structures for the following compounds.(a) 3-benzyl-4-bromohexane , 4,4-dimethylcyclohexene(b) trans-4,5-dibromohex-2-ene , cis-1,1-dibromo-2-ethyl-2,3-dimethylcyclobutanearrow_forward25. What is the systematic name for a compound with the following structure? CH2-Br CH3-CH2-CH-CH3 (A) 2-bromoisopentane (B) 1-bromo-2-methylbutane (C) 2-(bromomethyl)butane (D) 2-bromo-2-methylbutanearrow_forward
- Draw structural formulas for the following the compounds: (a) Cis-1,3-diphenylcyclohexane (b) 5-phenylpentanoic acid (c) 3,4-dibromo-N,N-dimethylanilinearrow_forwardDraw structures corresponding to the following IUPAC names: (a) 2-Methyl-1,5-hexadiene (b) 3-Ethyl-2,2-dimethyl-3-heptene (c) 2,3,3-Trimethyl-1,4,6-octatriene (d) 3,4-Diisopropyl-2,5-dimethyl-3-hexenearrow_forwardDraw structures (these may be either skeletal or displayed formulae) for the followingmolecules: (a) 2-methylbutanal(b) 1,6-diaminohexane(c) 1,1,2-trichloroethane(d) 2-methylcyclopentanone(e) but-2-ene-1-olarrow_forward
- H,C-CH-CH-CH, CHO (a) 3-ethyl-2-methybutanal (b) 2, 3-dimethylpentanal (c) 2-ethyl-3-methylbutanal (d) 2-ethyl-3-methylbutan-3-al CHO 2. The IUPAC name of is Br (a) 2-methyl-3-bromohexanal (b) 3-bromo-2-methylbutanal (c) 2-methyl-3-bromobutanal (d) 3-bromo-2-methylpentanal 3. The IUPAC name of the compound HO- isarrow_forwardPlease draw: (a) trans-2,3-dibromo-2-hexene (b) 1-ethyl-3-methylcyclohexane (c) 4-isopropyl-3-octanolarrow_forward34) The product formed by the reaction of toluene with chlorine in the presence of sunlight is: (a) o-chlorotoluene (b) 2,4-dichlorotoluene (c) p-chlorotoluene (d) Benzylchloridearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





