
(a)
Interpretation:
The given amine has to be classified as primary, secondary, or tertiary amine.
Concept Introduction:
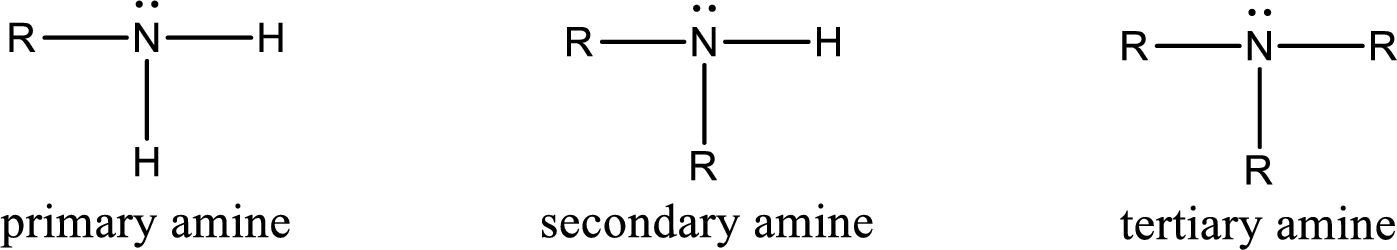
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the
Amides are also organic derivative. In an amide, the nitrogen atom is bonded to a carbonyl group. The general structural formula of amide can be given as shown below,
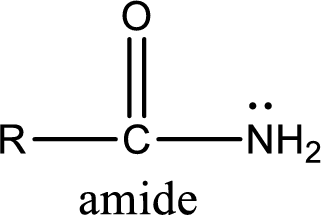
The difference between amine and amide is that in amine, the nitrogen atom is bonded to a hydrocarbon chain. In case of amides, the nitrogen atom is bonded to a carbonyl group.
(b)
Interpretation:
The given amine has to be classified as primary, secondary, or tertiary amine.
Concept Introduction:
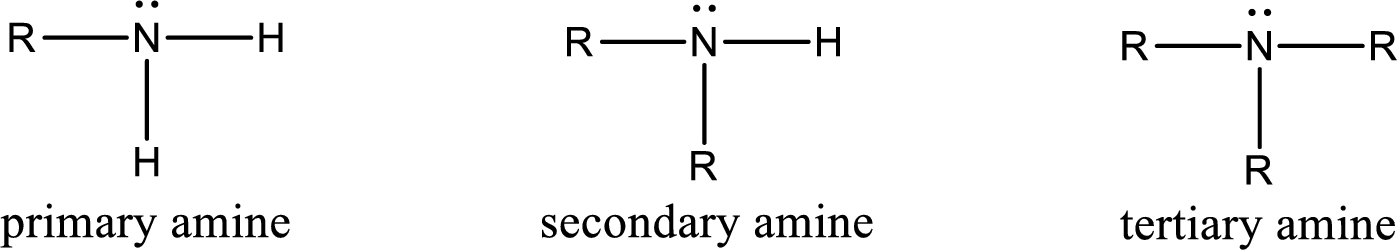
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
Amides are also organic derivative. In an amide, the nitrogen atom is bonded to a carbonyl group. The general structural formula of amide can be given as shown below,
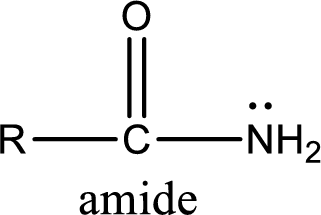
The difference between amine and amide is that in amine, the nitrogen atom is bonded to a hydrocarbon chain. In case of amides, the nitrogen atom is bonded to a carbonyl group.
(c)
Interpretation:
The given amine has to be classified as primary, secondary, or tertiary amine.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
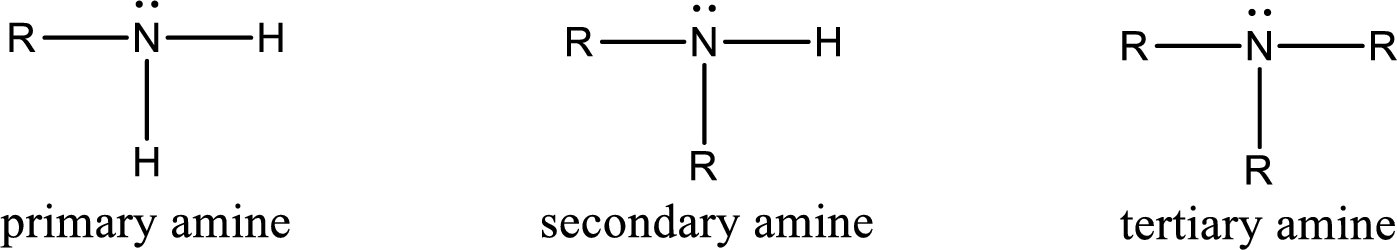
Amides are also organic derivative. In an amide, the nitrogen atom is bonded to a carbonyl group. The general structural formula of amide can be given as shown below,
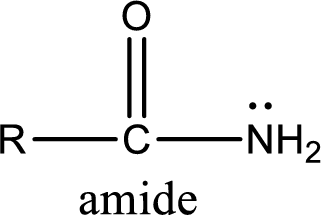
The difference between amine and amide is that in amine, the nitrogen atom is bonded to a hydrocarbon chain. In case of amides, the nitrogen atom is bonded to a carbonyl group.
(d)
Interpretation:
The given amine has to be classified as primary, secondary, or tertiary amine.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
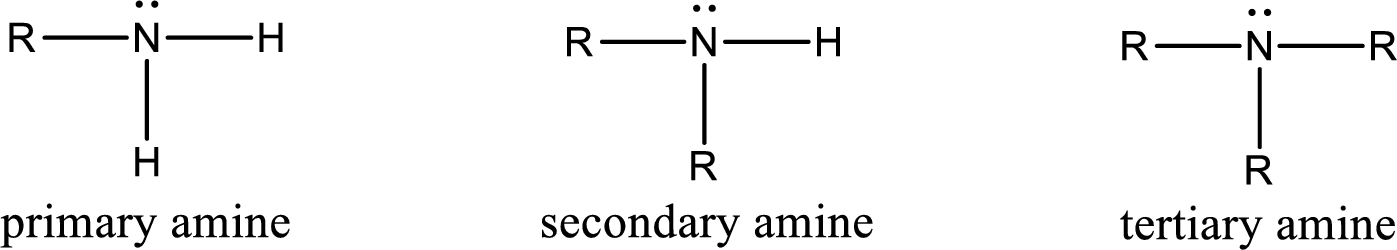
Amides are also organic derivative. In an amide, the nitrogen atom is bonded to a carbonyl group. The general structural formula of amide can be given as shown below,
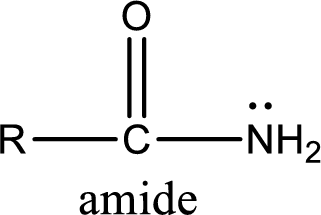
The difference between amine and amide is that in amine, the nitrogen atom is bonded to a hydrocarbon chain. In case of amides, the nitrogen atom is bonded to a carbonyl group.
Trending nowThis is a popular solution!

Chapter 17 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
- Indicate whether or not each of the following compounds contains an amine functional group?arrow_forwardGive each of the following amines an IUPAC name: a. b. c.arrow_forwardClassify the following compounds as a primary, secondary or tertiary amine. Be sure to label your answers clearly and briefly explain your reasoning. H NH2 N- II IIarrow_forward
- Classify each of the following amines as primary, secondary or tertiary.arrow_forwardA secondary amine has an amino group attached to a secondary carbon. True Falsearrow_forwardFollowing amines can be classified as --- and ---, respectively. H,N-CH3 H3C-CH3 primary amine, secondary amine O secondary amine, ammonium salt O secondary amine, primary amine O secondary amine, tertiary aminearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



