
Concept explainers
Draw the structure for each of the following:
- a. isobutyraldehyde
- b. 4-hexenaI
- c. diisopentyl
ketone - d. 3-methylcyclohexanone
- e. 2.4-pentanedione
- f. 4-bromo-3-heptanone
- g. γ-bromocaproaldehyde
- h. 2-ethylcyclopentanecarbaldehyde
- i. 4-methyl-5-oxohexanal
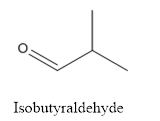
(a)
Interpretation:
The structure of Isobutyraldehyde has to be drawn.
Answer to Problem 53P
The structure of Isobutyraldehyde is given below:
Explanation of Solution
The compound has aldehyde group that is attached to the isobutyl group.
The structure of isobutyraldehyde is as follows.

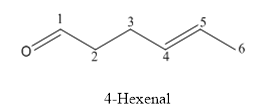
(b)
Interpretation:
The structure of 4-hexenal has to be drawn.
Answer to Problem 53P
The structure of 4-hexenal is given below:
Explanation of Solution
The compound has aldehyde functional group and double bond at the 4th position of the compound.
The structure is as follows.

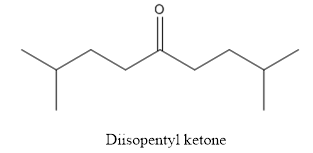
(c)
Interpretation:
The structure of diisopentyl ketone has to be drawn.
Answer to Problem 53P
The structure of diisopentyl ketone is given below:
Explanation of Solution
The compound has ketone functional group. Two isopentyl groups are attached to the keto group.
The structure of diisopentyl ketone is as follows.

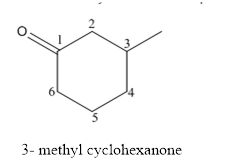
(d)
Interpretation:
The structure of 3-methylcyclohexanone has to be drawn.
Answer to Problem 53P
The structure of 3-methylcyclohexanoneis given below:
Explanation of Solution
The compound has ketone functional and group and it is called as cyclohexanone and it has one methyl group at 3rd position of the ring.
The structure of 3-methyl cyclohexanone is as follows.

(e)
Interpretation:
The structure of 2,4-pentanedione has to be drawn.
Answer to Problem 53P
The structure of 2,4-pentanedione is given below:
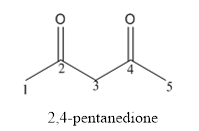
Explanation of Solution
The compound has two keto functional groups and these are attached to the pentane carbon chain.

(f)
Interpretation:
The structure of 4-bromo-3-heptanone has to be drawn.
Answer to Problem 53P
The structure of 4-bromo-3-heptanone is given below:
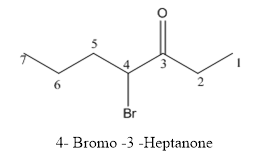
Explanation of Solution
The compound has keto functional group and it is attached to the heptanes carbon chain. At the 4th position of the carbon chain has bromine attachment.

(g)
Interpretation:
The structure of
Answer to Problem 53P
The structure of
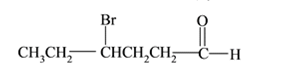
Explanation of Solution
Compound has aldehyde functional group and at the
The structure of
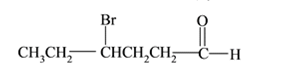
(h)
Interpretation:
The structure of 2-ethylcyclopentanecarbaldehyde has to be drawn.
Answer to Problem 53P
The structure of 2-ethylcyclopentanecarbaldehyde is given below:
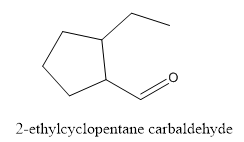
Explanation of Solution
In this compound, pentane ring has ethyl group and aldehyde functional group.
The structure of 2-ethylcyclopentane carbaldehyde.

(i)
Interpretation:
The structure of 4-methyl-5-oxohexanal has to be drawn.
Answer to Problem 53P
The structure of 4-methyl-5-oxohexanal is givenbelow:
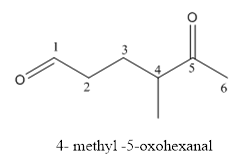
Explanation of Solution
The compound has aldehyde functional group at the 5th position of the carbon chain as keto group it is named as oxo-because it is referred to an attachment.
The structure of 4- methyl-5-oxohexanal.

Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry (8th Edition)
- Which of these is the best use of a volumetric flask? measuring how much liquid it contains delivering a precise amount of liquid to another container holding solutions making solutions of precise concentrationarrow_forwardYou're competing on a Great British television game show, and you need to bake a cake. The quantity for each ingredient is given in grams, but you haven't been given a kitchen scale. Which of these properties would correlate with the mass of a baking ingredient like eggs or milk? Check all that apply. depth of color viscosity volume densityarrow_forwardDraw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forward
- Is molecule 6 an enantiomer?arrow_forwardShow work. Don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forward
- Show work. don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardUse the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. || |II***** Molecule 1 | Molecule 4 none of the above Molecule 2 Molecule 3 Х mm... C ---||| *** Molecule 5 Molecule 6arrow_forwardis SiBr4 Silicon (IV) tetra Bromine? is KClO2 potassium dihypochlorite ?arrow_forward"יוון HO" Br CI Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 Br Br Br HO OH H CI OH ✓ Molecule 4 Molecule 5 Molecule 6 CI Br יייון H Br OH OH CI Br ☐ none of the above × Garrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





