
Concept explainers
(a)
Interpretation:
The product formed in the reaction of
Concept Introduction:
Potassium dichromate is a good oxidizing agent and it has the tendency to oxidize alcohols to
Answer to Problem 41P
Explanation of Solution
Aldehydes when made to react with potassium dichromate are known to form the corresponding carboxylic acids. The reaction of hexanal in the presence of potassium dichromate will result in the formation of hexanoic acid as shown below in the chemical equation:
Hence the reaction of hexanal with potassium dichromate results in the formation of the corresponding
(b)
Interpretation:
The product formed in the reaction of

Concept Introduction:
Potassium dichromate is a good oxidizing agent and it has the tendency to oxidize alcohols to aldehydes and aldehydes to carboxylic acids as well. An oxidizing agent is a chemical species which has the tendency to oxidize other to possible higher oxidation states by making them loose electron and thus itself gets reduced.
Answer to Problem 41P
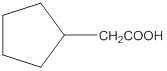
Explanation of Solution
Aldehydes when made to react with potassium dichromate are known to form the corresponding carboxylic acids. The reaction of 2-cyclopentylacetaldehyde in the presence of potassium dichromate will result in the formation of 2-cyclopentanoic acid as shown below in the chemical equation:
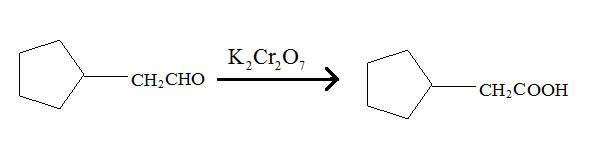
Hence the reaction of 2-cyclopentylacetaldehyde with potassium dichromate results in the formation of the corresponding carboxylic acid which is 2-cyclopentylacetic acid.
(c)
Interpretation:
The product formed in the reaction of
Concept Introduction:
Potassium dichromate is a good oxidizing agent and it has the tendency to oxidize alcohols to aldehydes and aldehydes to carboxylic acids as well. An oxidizing agent is a chemical species which has the tendency to oxidize other to possible higher oxidation states by making them loose electron and thus itself gets reduced.
Answer to Problem 41P
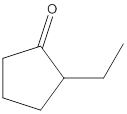
No product formed.
Explanation of Solution
The given compound is 2-ethylcyclopentanone and it cannot react with potassium dichromate as it contains ketone as a
(d)
Interpretation:
The product formed in the reaction of
Concept Introduction:
Potassium dichromate is a good oxidizing agent and it has the tendency to oxidize alcohols to aldehydes and aldehydes to carboxylic acids as well. An oxidizing agent is a chemical species which has the tendency to oxidize other to possible higher oxidation states by making them loose electron and thus itself gets reduced.
Answer to Problem 41P
Explanation of Solution
The given structure clearly shows that this compound is hexanol. It is an aliphatic alcohol and it will react with potassium reagent to form the corresponding aldehyde which will further get oxidized and the reaction will finally result in the formation of a carboxylic acid.
Want to see more full solutions like this?
Chapter 16 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- Classify each alkyl halide as 1°, 2°, or 3°. CH3 c. CHg-C-CHCH3 ČH3 ČI CH;CH2CH,CH,CH2-Br b. d. a.arrow_forward18. Ketone reduction Dicyclohexyl ketone Reduce the ketone. 1. NaBH4, ethanol 2. H3O+ H OH Dicyclohexylmethanol (88%) (a 2° alcohol)arrow_forward52. What is the major product of this reaction? 1) LDA, cold 2) CH3B. a b. d. OHarrow_forward
- Draw the organic product(s) formed when CH3CH2CH2OH is treated with each reagent. a.H2SO4 b.NaH c.HCl + ZnCl2 d.HBr e.SOCl2, pyridine f.PBr3 g.TsCl, pyridine h. [1] NaH; [2] CH3CH2Br [1] i.TsCl, pyridine; [2] NaSH j.POCl3, pyridinearrow_forwardDoes the equilibrium favor the reactants or products in each substitution reaction? a. CH;CH2-NH2 Br CH;CH2-Br + "NH2 b. "CN CN + I-arrow_forwardDraw the products formed when each compound is treated with CH;CH,COCI, AICI3. CH(CH3)2 N(CH)2 Br CH3 CH(CH)2 a. b. C. d. е.arrow_forward
- Draw the products of each reaction. OH он CH3 Cl2 FeCla Cl2 a. b. С. FeCl,arrow_forwardWhich is the major organic product of this reaction? A. B. 4 ОА O OB O C COD AICI3 benzene C. D.arrow_forward• Whał arc the IUPAC namar of the ff. Carboxylic acias? a. COOH COOH CH3 b. I f. .COOH CHJCH2 ÇHCHCOOH CH3 NO2 tON C. COOH ноос d. COOHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





