
Concept explainers
16-6 Answer true or false.
- te/7-Butylamine is a 3°
amine .
(a)
Interpretation:
To analyse whether the given statement: tert-Butylamine is
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 16.6P
The statement is false.
Explanation of Solution
The structure of a tert-butylamine is given below:
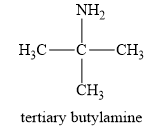
In a tert-butylamine, the carbon atom is bonded to three methyl groups, while nitrogen is attached to only one carbon group. Therefore, the tert-butyl amine is a primary amine. Therefore, this statement is false.
(b)
Interpretation:
To analyse whether the given statement about the aromatic amine is true or not.
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 16.6P
The statement is true.
Explanation of Solution
If in an amine, the nitrogen is directly bonded to one or more aryl groups or aromatic rings, and then the amine is known as an aromatic amine.
For example, aniline is an aromatic amine, as it is attached to one aromatic ring benzene. The structure of aniline is given below:
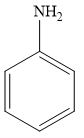
Therefore, the given statement is true.
(c)
Interpretation:
To analyse whether the given statement: In a heterocyclic amine, the main nitrogen is the part of the ringis true or not.
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 16.6P
The statement is true.
Explanation of Solution
In a heterocyclic amine, the carbon group of the ring structure is replaced by the nitrogen atom. For example, in pyridine, the nitrogen atom replaces one —CH group of the benzene ring.
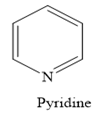
Thus, the heterocyclic amine is an amine in which nitrogen is one of the atoms of a ring. Therefore, this statement is true.
(d)
Interpretation:
To analyze whether the given statement: The NH4+ and CH4have the same number of valence electrons and both have tetrahedral geometry as per the VSEPR model is true or not.
Concept Introduction:
The VSEPR (Valence-shell electron pair repulsion) model predicts the shape of the molecules or ions by identifying the position of atoms connected to the central atom.
Answer to Problem 16.6P
The statement is true.
Explanation of Solution
The valence shell electron pair repulsion (VSEPR) theory determines the shapes and the geometry of a molecule. In a CH4 molecule, one carbon atom is bonded to four hydrogen atoms with a single bond. In NH4+, one nitrogen atom is singly bonded to four hydrogen atoms.
According to the VSEPR model, each bond represents a pair of electrons. Since both compounds have four bonds around the central atom, they contain eight valence electrons. Also, according to the VSEPR model, these four bonding regions are arranged in a tetrahedral manner so that they are as far away from one another as possible, giving the molecule a tetrahedral geometry. Therefore, this statement is true.
(e)
Interpretation:
To analyze whether the given statement: There are four constitutional isomers with the molecular formulaC3H9N, is true or not.
Concept Introduction:
Constitutional isomers are those compounds which have same molecular formula, but they differ in the arrangement of atoms in the molecules.
Answer to Problem 16.6P
The statement is true.
Explanation of Solution
The molecular formula is given as C3H9N. Therefore, the possible constitutional isomers for this molecular formula are given below:
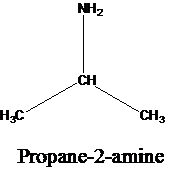
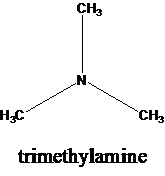
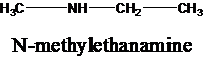
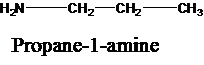
Thus, there are four constitutional isomers possible with the molecular formula C3H9N. Therefore, this statement is true.
Want to see more full solutions like this?
Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
- A reaction between a Lewis Acid and a Lewis Base is given below: Complete the reaction and identify the structure of the missing product (only one). Use curly arrows to show electron transfers. Show all formal charges (if any). Cl~Al _CI ? Clarrow_forwardDetermine whether the following lewis structure dot of acetylsalicylic acid, C9H8O4 is valid or no and explain why? H :O: 08-11 || H || bobno C Holo C H Thois H O-C=C-H || :O: H obolin nogoarrow_forwardWhat is the meaning of the term tertiary (3°) when it is used to classify amines? Draw a structural formula for the one tertiary (3°) amine known as Hünig’s base (N,N-diisopropylethylamine).arrow_forward
- Which of the following are examples of isobars? S-40 & CI-40 S-32 & P-31 Na-37 & K-39 C-12 & C-14arrow_forwardMethyl isocyanate, CH3 -N= C = O, is used in the industrial synthesis of a type of pesticide and herbicide known as a carbamate. As a historical note, an industrial accident in Bhopal, India, in 1984 resulted in leakage of an unknown quantity of this chemical into the air. An estimated 200,000 people were exposed to its vapors, and over 2000 of these people died. Q.) Write a Lewis structure for methyl isocyanate and predict its bond angles. What is the hybridization of its carbonyl carbon? Of its nitrogen atom?arrow_forwardDraw the product formed when the Lewis acid (CH;CH),C* reacts with each Lewis base: (a) H20; (b) CH3OH; (c) (CH3)20; (d) NH3; (e) (CH3)2NH.arrow_forward
- One family of disinfection byproducts that result from the reaction of chloramines with natural organic matter in water are called the halomethanes. These are molecules where the hydrogen atoms in methane (CH4) have been replaced with atoms from group 7 of the periodic table (i.e. halogens). Draw and submit pictures of the Lewis structures for each of the following halomethanes: CH3Cl CCI CBrCizarrow_forwardPart 1: Figures 1.1-1.11 belong to some of the organic solvents you will be using this semester. Please write down the STRUCTURE of the compound in the box: ¹H-NMR ¹H-NMR ¹H-NMR EME ¹H-NMR 3 Figure 1.1: The structure of this Tertiary amine (molecular formula C6H15N) is: Figure 1.2: The structure of the Secondary amine is (molecular weight 73): Figure 1.3: The structure of this primary alcohol (C4H10O) is: Figure 1.4: The structure of this ester (C4H8O2) is: Page 1 of 4arrow_forwardDraw the attraction between a water molecule and a nitrite ion, NO2-1 First, draw one molecule and add the partial charges where needed - use the ΔEN to determine the types of bonds. Then, draw the second molecule so that the δ+ on one molecule lines up across from the δ- on the other. Since we can't draw the molecules here you will answer questions about the drawings that you made on the homework worksheet. NO2-1 has ________ bonds with a ΔEN = ________ . NO2-1 has 3 REDs and is symmetrical/asymmetrical (answer is ________ ). However, NO2-1 has one electron more than its protons making it _________ text. . H2O has 2 ___________ bonds with a ΔEN = __________ . H2O has 4 REDs and is symmetrical/asymmetrical (answer is _______ ) making it a polar/nonpolar (answer is ________ ) molecule. Each H has a ___________ charge and the O has a ________ charge. The strongest possible attractive force between these two molecules is _________ .arrow_forward
- ontent/2973671/viewContent/1868045/View a) CH;SH + H,0 CH;S + H3O* b) CH;CH,NH, + H2O CH;CH,NH, + OHarrow_forwardSelect the bonds in the 3D representation of methyl urethane below that are multiple bonds. (Hint: Consider the number of bonds attached to an atom in the image compared to the normal valence of that atom. In a valid structure, the number of bonds to an atom will equal the valence of that atom.) • Gray = C: white = H; red = 0; blue = N; dark green = Cl; brown = Br; light green = F; purple = 1; yellow = S: orange = P. • Double click to select bonds. You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens). ●arrow_forward1) Answer the following questions using the table below: Acid Benzoic Acid, C6H5CO2H Benzylammonium ion, C6HSCH2NH3* Chloroacetic acid, CICH2CO2H Hydrogen Sulfide, H2S Ka 6.31 x 10-5 4.47 x 10-10 1.35 x 10-3 1.00 x 10-7 Nitrous Acid, HNO2 4.47 x 104 a) Which is the strongest acid? b) Which acid has the highest pH, and what is the chemical formula of the conjugate base? c) Write the reaction of nitrous acid (HNO2) acting as a Brønsted-Lowry acid in water and write the Ka expression.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



