
To sketch:
A titration curve for the titration of
a)
b)
c)
d)
Answer to Problem 16.45QA
Solution:
a)
b)
c)
d)
Explanation of Solution
1) Concept:
The given titration is the titration of a weak acid and a strong base. The volume of the sample of the acid and its molarity is given. We can find the initial moles of the acid present using these values. When
The initial moles of
Where,
The
The relation for
2) Formula:
i)
ii)
iii)
iv)
v)
3) Given:
i) Molarity of
ii) Volume of
iii) Molarity of
iv)
4) Calculation:
Calculating the initial moles of
a) Calculating the pH of solution when
Calculating the moles of
Set up the RICE table to determine the reacted moles of
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Calculating the concentration of
Plug in the values in the Henderson–Hasselbalch equation,
b) Calculating the pH of solution when
Calculating the moles of
Set up the RICE table to determine the reacted moles of
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Calculating the concentration of
Plug in the values in the Henderson–Hasselbalch equation,
c) Calculating the pH of solution when
Calculating the moles of
Set up the RICE table to determine the reacted moles of
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Calculating the concentration of
Plug in the values in the Henderson–Hasselbalch equation,
d) Calculating the pH of solution when
Calculating the moles of
Set up the RICE table to determine the reacted and remaining moles of
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Since the acid and added base have reacted completely, and an equivalent amount of conjugate base has formed, this represents the equivalence point.
Calculating the concentration of
Set up RICE table to calculate the
| Reaction | + | + | |||||
| Initial | 0 | 0 | |||||
| Change | |||||||
| Equilibrium |
Calculating
Calculating the
A titration curve for the titration of
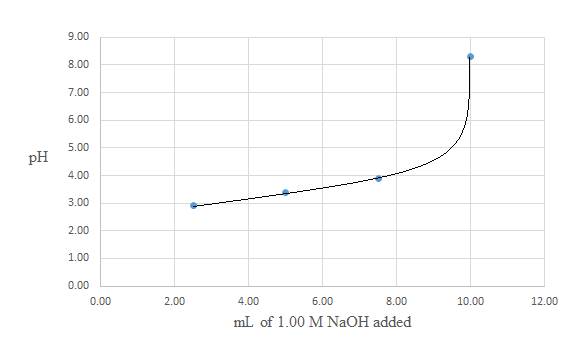
Conclusion:
In the titration of a weak acid and a strong base, as you increase the volume of the strong base, the
Want to see more full solutions like this?
Chapter 16 Solutions
CHEM:ATOM FOC 2E CL (TEXT)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





