
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Chapter 16, Problem 16.40P
Interpretation Introduction
Interpretation: The given diene is to be ranked in increasing order of heat of hydrogenation.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
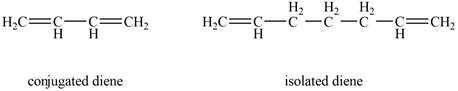
Figure 1
The stability of conjugated diene is more than isolated diene. Due to which the heat of hydrogenation is smaller for conjugated diene, whereas heat of hydrogenation for isolated diene is larger.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used Ai solution
Don't used Ai solution
Please correct answer and don't used hand raiting
Chapter 16 Solutions
Organic Chemistry
Ch. 16 - Prob. 16.1PCh. 16 - Prob. 16.2PCh. 16 - Problem 16.3 Draw a second resonance structure for...Ch. 16 - Prob. 16.4PCh. 16 - Problem 16.5 Farnesyl diphosphate is synthesized...Ch. 16 - Prob. 16.6PCh. 16 - Prob. 16.7PCh. 16 - Prob. 16.8PCh. 16 - Problem 16.9 Determine the hybridization of the...Ch. 16 - Problem 16.10 Draw the structure consistent with...
Ch. 16 - Problem 16.11 Neuroprotectin D1 (NPD1) is...Ch. 16 - Problem 16.12 Using hybridization, predict how the...Ch. 16 - Problem 16.13 Use resonance theory to explain why...Ch. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - Problem 16.16 Draw the products formed when each...Ch. 16 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 16 - Prob. 16.18PCh. 16 - Problem 16.19 Draw the product formed when each...Ch. 16 - Prob. 16.20PCh. 16 - Prob. 16.21PCh. 16 - Problem 16.22 Rank the following dienophiles in...Ch. 16 - Prob. 16.23PCh. 16 - Prob. 16.24PCh. 16 - Problem 16.25 What diene and dienophile are needed...Ch. 16 - Prob. 16.26PCh. 16 - Problem 16.27 Which compound in each pair absorbs...Ch. 16 - Prob. 16.28PCh. 16 - 16.29 Name each diene and state whether the...Ch. 16 - Prob. 16.30PCh. 16 - 16.31 Which of the following systems are...Ch. 16 - 16.32 Draw all reasonable resonance structures for...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - 16.35 Explain why the cyclopentadienide anion A...Ch. 16 - Prob. 16.36PCh. 16 - 16.37 Draw the structure of each compound.
a. in...Ch. 16 - Prob. 16.38PCh. 16 - 16.39 Label each pair of compounds as...Ch. 16 - Prob. 16.40PCh. 16 - 16.41 Draw the products formed when each compound...Ch. 16 - Prob. 16.42PCh. 16 - 16.43 Treatment of alkenes A and B with gives the...Ch. 16 - 16.44 Draw a stepwise mechanism for the following...Ch. 16 - Prob. 16.45PCh. 16 - 16.46 Explain, with reference to the mechanism,...Ch. 16 - Prob. 16.47PCh. 16 - Prob. 16.48PCh. 16 - Prob. 16.49PCh. 16 - Prob. 16.50PCh. 16 - Prob. 16.51PCh. 16 - Prob. 16.52PCh. 16 - 16.53 Diels–Alder reaction of a monosubstituted...Ch. 16 - Prob. 16.54PCh. 16 - 16.55 Devise a stepwise synthesis of each compound...Ch. 16 - Prob. 16.56PCh. 16 - 16.57 A transannular Diels–Alder reaction is an...Ch. 16 - Prob. 16.58PCh. 16 - Draw a stepwise mechanism for the following...Ch. 16 - Prob. 16.60PCh. 16 - Prob. 16.61PCh. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - 16.65 The treatment of isoprene with one...Ch. 16 - 16.66 The treatment of with forms B (molecular...Ch. 16 - Prob. 16.67PCh. 16 - Prob. 16.68PCh. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Prob. 16.73PCh. 16 - Prob. 16.74PCh. 16 - Prob. 16.75P
Knowledge Booster
Similar questions
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
