
(a)
Interpretation:
The structure of
Concept introduction:
Answer to Problem 16.2P
The structure of
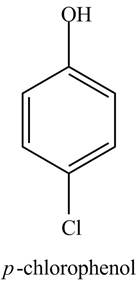
Explanation of Solution
The aromatic compound,

Figure 1
The structure of
(b)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
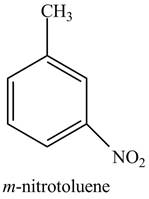
Explanation of Solution
The aromatic compound,
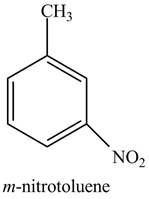
Figure 2
The structure of
(c)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
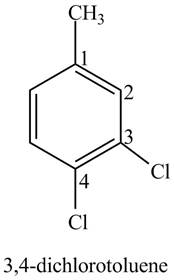
Explanation of Solution
The aromatic compound,

Figure 3
The structure of
(d)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
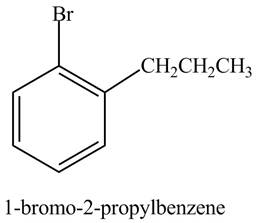
Explanation of Solution
The aromatic compound,
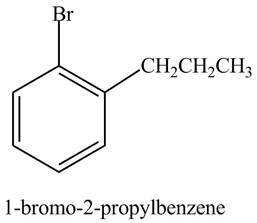
Figure 4
The structure of
(e)
Interpretation:
The structure of methyl phenyl ether is to be stated.
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of methyl phenyl ether is shown below.
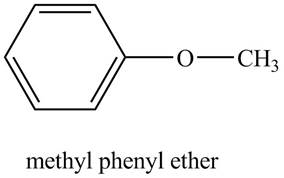
Explanation of Solution
The compound, methyl phenyl ether contains a methyl group and a phenyl ring as a substituent attached to the oxygen atom of the ether

Figure 5
The structure of methyl phenyl ether is shown in Figure 5.
(f)
Interpretation:
The structure of benzyl methyl ether is to be stated.
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of benzyl methyl ether is shown below.
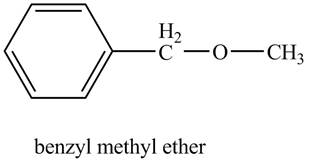
Explanation of Solution
Benzene ring with a methyl substituent is known as a benzyl group. The compound, benzyl methyl ether contains a methyl group and a benzyl group as a substituent on the oxygen atom of the ether functional group as shown below.
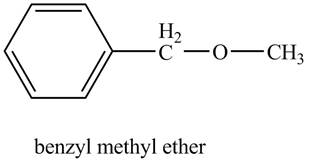
Figure 6
The structure of benzyl methyl ether is shown in Figure 6.
(g)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
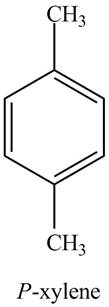
Explanation of Solution
The common name of

Figure 7
The structure of
(h)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
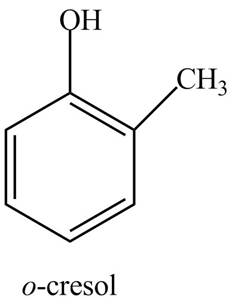
Explanation of Solution
The common name of
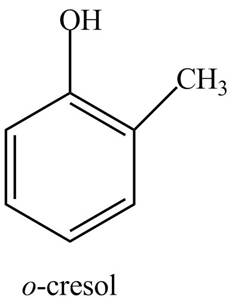
Figure 8
The structure of
(i)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
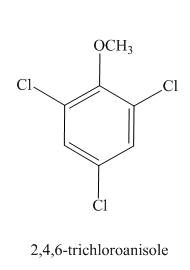
Explanation of Solution
The aromatic compound,

Figure 9
The structure of
Want to see more full solutions like this?
Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
- Draw the structure of the following compounds all showing C and H atoms.(a) 2-methyl -3-iso propyl heptanes(b) Dicyclopropyl methane.arrow_forwardWrite structural formulas for the following compounds (includes both old- and new-style names).(a) 2-octyne (b) ethylisopentylacetylene (c) ethynylbenzene(d) cyclohexylacetylene (e) 5-methyl-3-octyne (f) trans-3,5-dibromocyclodecyne(g) 5,5-dibromo-4-phenylcyclooct-1-yne (h) (E)-6-ethyloct-2-en-4-yne (i) 1,4-heptadiyne(j) vinylacetylene (k) (S)-3-methyl-1-penten-4-ynearrow_forwardWrite structural formulas for the following compounds (includes both old- and new-style names).(a) 2-octyne (b) ethylisopentylacetylene (c) ethynylbenzene(d) cyclohexylacetylene (e) 5-methyl-3-octyne (f) trans-3,5-dibromocyclodecyne(g) 5,5-dibromo-4-phenylcyclooct-1-yne (h) (E)-6-ethyloct-2-en-4-yne (i) 1,4-heptadiynearrow_forward
- Describe how would you distinguish the following pairs, (a) Benzene and cyclohexane (b) Phenol and toluene (c) Phenol and benzoic acidarrow_forwardUsing cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctene (b) cyclooctane (c) trans-1,2-dibromocyclooctane(d) cyclooctanone (e) 1,1-dibromocyclooctane (f) 3-bromocyclooctene(g) cyclooctane-1,2-dionearrow_forward(b) Draw the structural formula for each of the following compounds. Lukis formula struktur bagi setiap sebatian berikut. (i) 3-isopropyl-3,4-dimethylhexanol (ii) 2-methyl-3-phenylprop-2-enal (iii) N-ethyl-N-methylbutanaminearrow_forward
- Using cyclohexane as your starting material, show how you would synthesize each of the following compounds. (Onceyou have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) bromocyclohexane (b) cyclohexenearrow_forwardUsing cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctene (b) cyclooctane (c) trans-1,2-dibromocyclooctanearrow_forward(d) How would you prepare any one of the following compounds from benzene? More than one step may be involved in each case. (a) (b) OH Br m-Bromo benzoic acid Phenyl acetic acidarrow_forward
- Write equations showing how 2-phenylethanol could be prepared from each of the following starting materials:(a) Bromobenzene (c) 2-Phenylethanal (C6H5CH2CHO)(b) Styrene (d) 2-Phenylethanoic acid (C6H5CH2CO2H)arrow_forward(a) Which of the following will NOT produce a carboxylic acid or carboxylate ion? 1-butanol + H2CrO4 2-butene + O3/H2O2 butanal + PCC butanal + H2CrO4 (b) Which of the following will NOT produce a carboxylic acid or carboxylate ion? 1-butanol + H2CrO4 2-butene + O3/H2O2 butanal + PCC butanal + H2CrO4arrow_forwardWrite a structural formula for each of the following compounds: (a) m-Chlorobenzoyl chloride (b) Trifluoroacetic anhydride (c) cis-1,2-Cyclopropanedicarboxylic anhydride (d) Ethyl cycloheptanecarboxylate (e) 1-Phenylethyl acetate (f) 2-Phenylethyl acetate (g) p-Ethylbenzamide (h) N-Ethylbenzamide (i) 2-Methylhexanenitrilearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





