
(a)
Interpretation:
Condensed structural formula has to be drawn for the given
Concept Introduction:
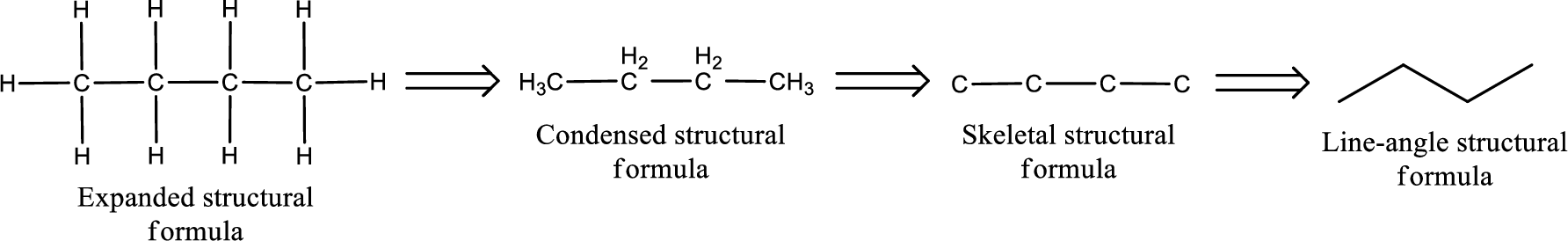
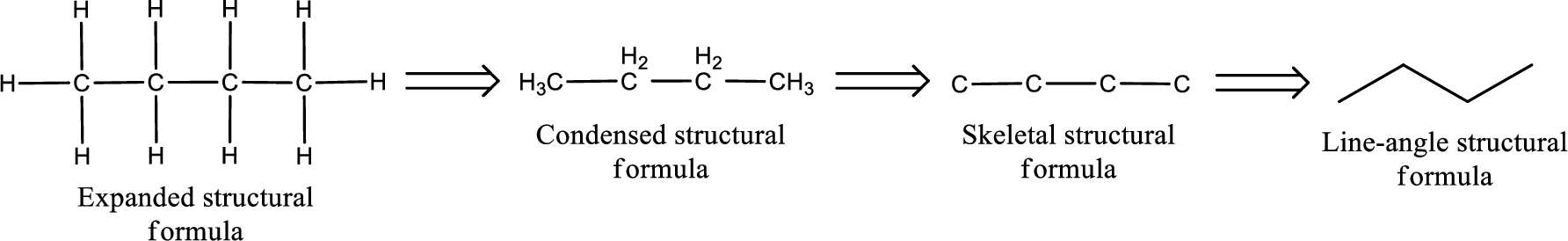
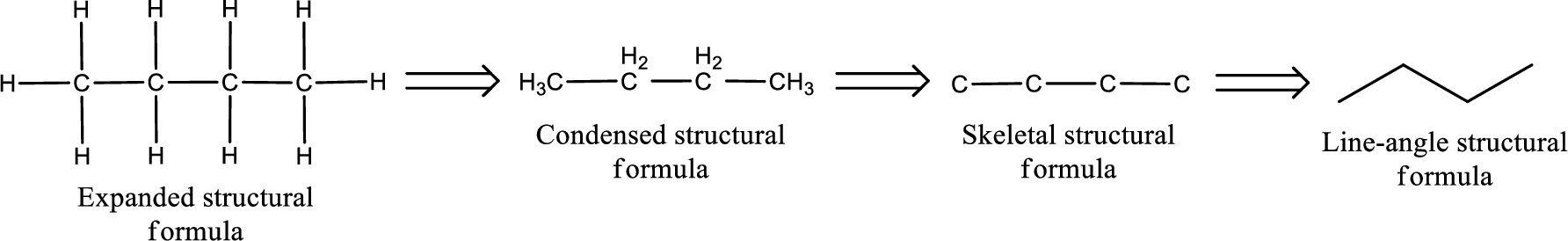
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(a)
Answer to Problem 16.29EP
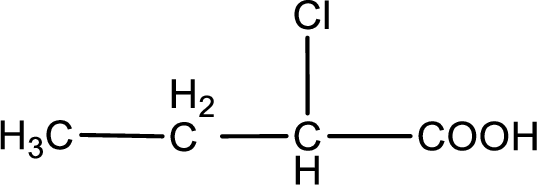
The condensed structural formula is,
Explanation of Solution
Given description about the carboxylic acid is
In the problem statement it is given how the carboxyl group has to be denoted. From the common name it is understood that the carboxylic acid is a four carbon monocarboxylic acid. The chlorine atom is substituted in the alpha carbon atom. Alpha carbon atom in carboxylic acid is the one that is attached to the carboxyl group. Therefore, the structure can be drawn as shown below,
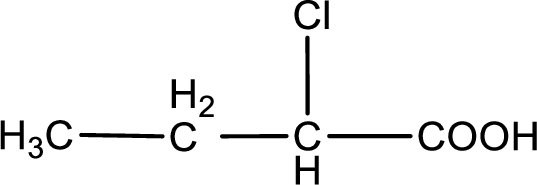
The condensed structural formula for the given carboxylic acid is drawn.
(b)
Interpretation:
Condensed structural formula has to be drawn for the given carboxylic acid.
Concept Introduction:
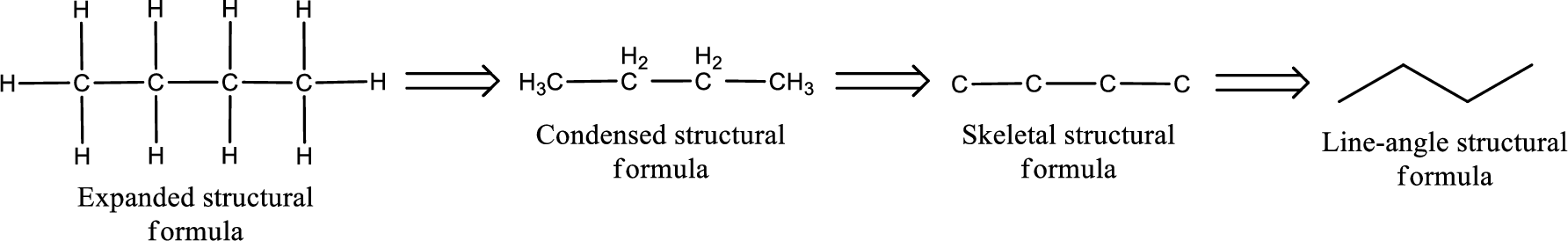
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(b)
Answer to Problem 16.29EP
The condensed structural formula is,
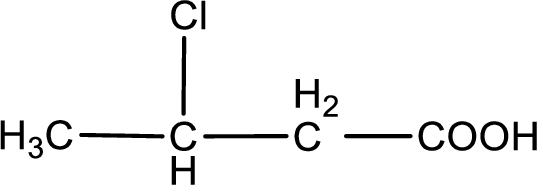
Explanation of Solution
Given description about the carboxylic acid is
In the problem statement it is given how the carboxyl group has to be denoted. From the common name it is understood that the carboxylic acid is a four carbon monocarboxylic acid. The chlorine atom is substituted in the beta carbon atom. Beta carbon atom in carboxylic acid is the one that is the second carbon atom from the carboxyl group. Therefore, the structure can be drawn as shown below,
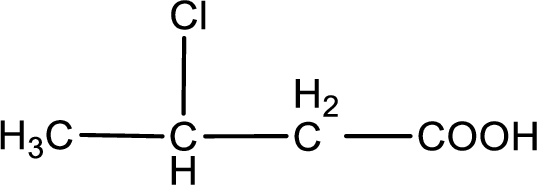
The condensed structural formula for the given carboxylic acid is drawn.
(c)
Interpretation:
Condensed structural formula has to be drawn for the given carboxylic acid.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(c)
Answer to Problem 16.29EP
The condensed structural formula is,
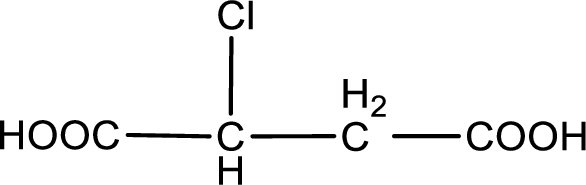
Explanation of Solution
Given description about the carboxylic acid is chlorosuccinic acid.
In the problem statement it is given how the carboxyl group has to be denoted. From the common name it is understood that the carboxylic acid is a four carbon dicarboxylic acid. The chlorine atom is substituted in the either of the two carbon atoms as both the carbon atoms present between the two carboxyl groups are identical. Therefore, the structure can be drawn as shown below,
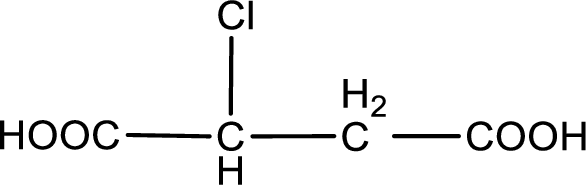
The condensed structural formula for the given carboxylic acid is drawn.
(d)
Interpretation:
Condensed structural formula has to be drawn for the given carboxylic acid.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
(d)
Answer to Problem 16.29EP
The condensed structural formula is,
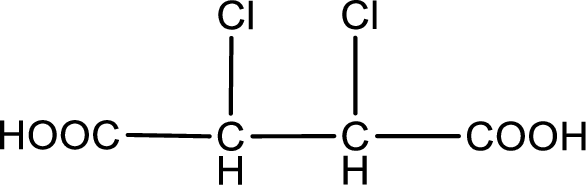
Explanation of Solution
Given description about the carboxylic acid is
In the problem statement it is given how the carboxyl group has to be denoted. From the common name it is understood that the carboxylic acid is a four carbon dicarboxylic acid. The chlorine atom is substituted in the alpha carbon atom and beta carbon atom. Alpha carbon atom in carboxylic acid is the one that is attached to the carboxyl group and the next carbon attached to the alpha carbon atom is the beta carbon atom. Therefore, the structure can be drawn as shown below,
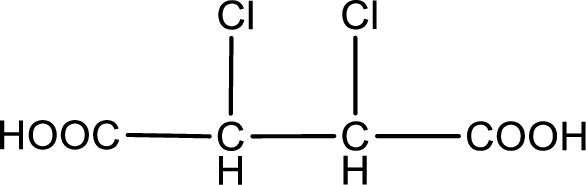
The condensed structural formula for the given carboxylic acid is drawn.
Want to see more full solutions like this?
Chapter 16 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- Ph heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forward
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





