
(a)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
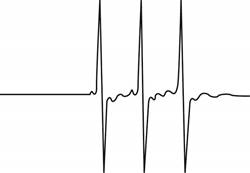
Figure 1
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Three different peaks with same intensity indicate that
Substitute the values of
The above equation is further simplified for the value of
Therefore, the spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(b)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
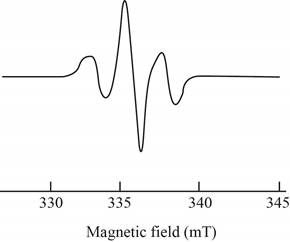
Figure 2
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Three peaks with different intensities indicate that two equivalent nuclei are present.
Substitute the values of
The above equation is further simplified for the value of
Therefore, spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(c)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
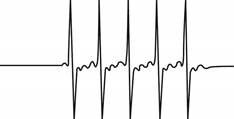
Figure 3
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Five different peaks with same intensity represents that
Substitute the values of
The above equation is further simplified for the value of
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(d)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
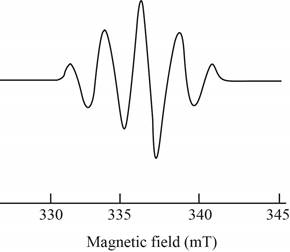
Figure 4
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Five different peaks with different intensities indicate that four equivalent nuclei are present.
Substitute the values of
The above equation is further simplified for the value of
Therefore, the spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Want to see more full solutions like this?
Chapter 16 Solutions
Bundle: Physical Chemistry, 2nd + Student Solutions Manual
- 6 of 15 Based on their corresponding nuclear spin number (reported within brackets), determine which of the following nuclei can be studied by NMR spectroscopy. Choose all correct answers. Please, be aware that incorrect answers will result in negative scores. O Nitrogen: 14N(1) O Fluorine: 19F(1/2) O Oxygen: 160(0) O Deuterium: 2H(1) O Carbon: 12c(0) O Proton: 'H(1/2) O Carbon 13: 13C(1/2) O Nitrogen 15: 15N(1/2)arrow_forwardTrue or False : The difference in energy, ΔE, between alfa (+1/2) and beta (–1/2) spin states of a proton is independent of the strength of the applied magnetic field. Could you explain detail?arrow_forwardPredict the spin-spin coupling (aka splitting) by providing the multiplicity (singlet, doublet, triplet, doublets of doublet, quartet. etc.) of Ha, Hb, Hc, and Hd of the molecules below. Please decide how many non-equivalent protons in each given molecule.arrow_forward
- Draw spin-spin splitting diagrams for the peaks in the NMR spectrum attached below.arrow_forwardProton nuclear magnetic resonance spectroscopy ('H-NMR) 1.(2) Which of the following statements about proton NMR spectroscopy is FALSE? (Circle/indicate) a) proton nmr requires a strong external magnetic field, and radio frequency transmitter and received b) the energies involved in proton nmr are so large, that the sample is destroyed in the process c) the kinds of information available are chemical shift, integrations, and spin-spin splitting d) the reference standard in proton nmr is tetramethylsilane, chemical shift = 0.0arrow_forwardPredict the multiplet you would expect for coupling to two equivalent spin-1 nuclei.arrow_forward
- 1. Answer BOTH parts a) and b). a) i) Explain the difference between spin-lattice relaxation (T₁) and spin-spin relaxation (T2). ii) Deuterated acetone, (CD3)2CO, is a common solvent for NMR spectroscopy. Sketch the 1³C{¹H} NMR spectrum of this solvent. Label and explain any coupling in your spectrum. iii) Predict the structure of the residual protio-solvent species observed in the ¹H NMR spectrum of (CD3)2CO. b) OH A B C max 260 nm 295 nm i) Suggest electronic transitions that can be attributed to the absorptions reported for compounds A and B, respectively. ii) With the aid of diagrams explain why B absorbs at a lower energy to A. iii) Explain why compound C possesses a charge transfer transition. iv) For compound C explain why changing solvent polarity influences the wavelength of the charge transfer absorption. -CEZarrow_forwardPredict 31PNMR spectrum of [Rh(PR3)Cl2]. Given phosphorus-31 is 100% abundant, rhodium is 100% abundant, all nuclear spins are (I=1/2).arrow_forwardBased on the IR stretching frequencies values listed choose the best molecule from the set that matches the information. (a) 3095, 2980, 2965, 1685, 1600, 1510 cm¹ (b) 3300 (br), 2980, 2970, 1715 cm¹ OH (c) 3300 (br), 2980, 2970 cm¹ - OH OH (d) 2980, 2970, 2850 (m), 2750 (m), 1715 cm³ NH e z Br OH OH Harrow_forward
- Q1 (a) Explain how the peaks in Infra-Red spectroscopy are generated and what information the peaks can provide about the molecule analysed.(250 words limit) 25% (b) Explain how interaction of Ultra-Violet / Visible light with a molecule produces an absorbance and hence a peak, indicating what parts of a molecule can absorb this radiation. (250 words limit) (25%)arrow_forwardHow to calculate spin of atomic nuclei for proton NMRarrow_forwardA scientist investigates the possibility of neutron spin resonance, and has available a commercial NMR spectrometer operating at 300 MHz for 1H nuclei. What is the NMR frequency of the neutron in this spectrometer? What is the relative population difference at room temperature? Which is the lower energy spin state of the neutron?arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning




