
Concept explainers
(a)
Interpretation:
To draw the structure of the given compounds.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the
(a)
Answer to Problem 48P
The structure of N,N-dimethylhexanamide is:
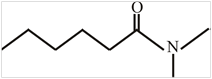
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is N,N-dimethylhexanamide. It indicates that six carbons are present in the compound. The suffix is ‘amide’ indicating the presence of an amide group
Thus, the structure is drawn as,

(b)
Interpretation:
To draw the structure of the given compounds.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(b)
Answer to Problem 48P
The structure of 3,3-dimethylhexanamide is:
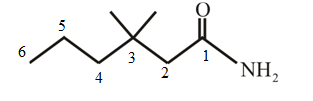
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is 3,3-dimethylhexanamide. It indicates that six carbons are present in the compound. The suffix is ‘amide’ indicating the presence of an amide functional group
Thus, the structure is drawn as,

(c)
Interpretation:
To draw the structure of the given compound.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(c)
Answer to Problem 48P
The structure of
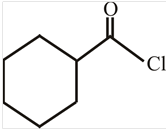
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(d)
Interpretation:
To draw the structure of the given compound.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(d)
Answer to Problem 48P
The structure of
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(e)
Interpretation:
To draw the structure of the given compounds.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(e)
Answer to Problem 48P
The structure of
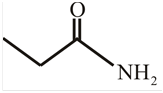
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(f)
Interpretation:
To draw the structure of the given compound.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(f)
Answer to Problem 48P
The structure of
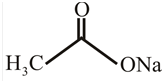
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(g)
Interpretation:
To draw the structure of the given compounds.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(g)
Answer to Problem 48P
The structure of
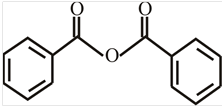
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
The suffix is ‘anhydride’ indicating the presence of indicating the presence of an acid anhydride group in which the
Thus, the structure is drawn as,

(h)
Interpretation:
To draw the structure of the given compound.
Concept introduction: The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(h)
Answer to Problem 48P
The structure of
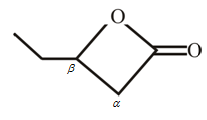
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(i)
Interpretation:
To draw the structure of the given compounds.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(i)
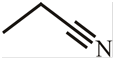
Answer to Problem 48P
The structure of 3-methylbutanenitrile is:
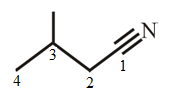
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is 3-methylbutanenitrile. It indicates that four carbons are present in the carbon skeleton of the compound. The suffix is ‘nitrile’ indicating the presence of a nitrile functional group. There is a methyl group on the third carbon in the chain. The numbering of carbons starts from the functional group side.
Thus, the structure is drawn as,

(j)
Interpretation:
To draw the structure of the given compound.
Concept introduction: The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(j)
Answer to Problem 48P
The structure of
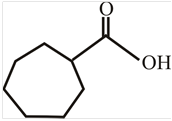
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(k)
Interpretation:
To draw the structure of the given compound.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
(k)
Answer to Problem 48P
The structure of
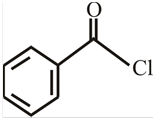
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

Want to see more full solutions like this?
Chapter 15 Solutions
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. < cleavage Bond A • CH3 + 26. t cleavage 2°C• +3°C• Bond C Cleavage CH3 ZC '2°C. 26. E Strongest 3°C. 2C. Gund Largest BDE weakest bond In that molecule a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest C bond Produces A Weakest Bond Most Strongest Bond Stable radical Strongest Gund produces least stable radicals b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 人 8°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. methyl radical •CH3 formed in bund A Cleavagearrow_forwardWhich carbocation is more stable?arrow_forward
- Are the products of the given reaction correct? Why or why not?arrow_forwardThe question below asks why the products shown are NOT the correct products. I asked this already, and the person explained why those are the correct products, as opposed to what we would think should be the correct products. That's the opposite of what the question was asking. Why are they not the correct products? A reaction mechanism for how we arrive at the correct products is requested ("using key intermediates"). In other words, why is HCl added to the terminal alkene rather than the internal alkene?arrow_forwardMy question is whether HI adds to both double bonds, and if it doesn't, why not?arrow_forward
- Strain Energy for Alkanes Interaction / Compound kJ/mol kcal/mol H: H eclipsing 4.0 1.0 H: CH3 eclipsing 5.8 1.4 CH3 CH3 eclipsing 11.0 2.6 gauche butane 3.8 0.9 cyclopropane 115 27.5 cyclobutane 110 26.3 cyclopentane 26.0 6.2 cycloheptane 26.2 6.3 cyclooctane 40.5 9.7 (Calculate your answer to the nearest 0.1 energy unit, and be sure to specify units, kJ/mol or kcal/mol. The answer is case sensitive.) H. H Previous Nextarrow_forwardA certain half-reaction has a standard reduction potential Ered +1.26 V. An engineer proposes using this half-reaction at the anode of a galvanic cell that must provide at least 1.10 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell. Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box.. Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. yes, there is a minimum. 1 red Πν no minimum Oyes, there is a maximum. 0 E red Dv By using the information in the ALEKS…arrow_forwardIn statistical thermodynamics, check the hcv following equality: ß Aɛ = KTarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward(11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





