
Interpretation:
The cell voltage at each volume of
Concept Introduction:-
Cell Voltage:
Cell voltage is the difference between cathode potential and anode potential.
The unreacted concentration in the solution is determined by
Ion-selective electrode:
An ion-selective electrode (ISE) best known as a specific ion electrode (SIE), is a sensor (or transducer) that transforms the activity of a specific ion dissolved in a solution into an electrical potential.
Ion-selective electrode obeys following equation.
Explanation of Solution
Given:
The titration reaction is a reduction reaction.
The cell voltage of the reaction is written as
Cell Voltage:
Cell voltage is the difference between cathode potential and anode potential.
Where,
Another titration reaction is
The solubility constant for
The given equivalence point is
The unreacted
At
At
At
At
After
At
At
At
All the obtained potential are plotted as,
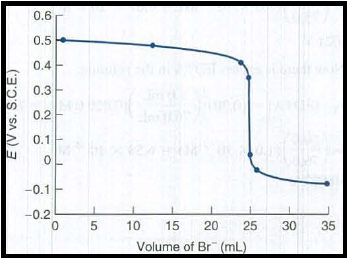
Figure 1
The cell voltage at each volume of
Want to see more full solutions like this?
Chapter 15 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





