
(a)
Interpretation:
The compound Propene exhibit geometric isomerism or not has to be given and two isomers has to be drawn and named for the given structure.
Concept Introduction:
The structure of the compound is given by its systematic name.
To give the structure from the name of the compound, the root name has to be identified. The root name indicates the number of carbon atoms present in the longest chain.
Then the functional group (suffix) has to be identified. It indicates whether any
The prefix of the name indicates the branched groups and their positions on the carbon chain.
The name of the compound is in the form
Prefix + Root + Suffix
The geometric isomers are said to be the isomers which shows different orientation of groups around a double bond. The geometric isomers are also known as cis-trans isomers.
When two similar or higher priority groups are attached to the carbon on same side, it is said to be cis-isomer.
When two similar or higher priority groups are attached to the carbon on opposite sides, it is said to be trans-isomer.
To exhibit the geometric isomerism, a molecule should have double bonded carbon atoms (
(a)
Explanation of Solution
The given compound is Propene.
The propene contains three carbon atoms in chain. As the suffix is –ene, it belongs to alkene group and contains a double bond. The position of the double bond in propene is in between first and second carbon atoms. The structure of propene is given as
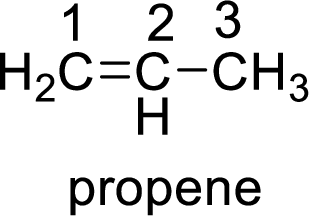
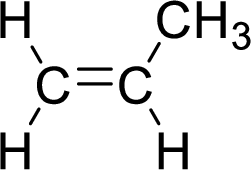
The given compound is propene. The given compound contains a double bond and each carbon in the double bond is bonded to two groups.
The geometric isomers are cis-isomer and trans-isomer. In cis-isomer, similar groups are attached to the carbon on same side and in trans-isomer, similar groups are attached to the carbon on opposite sides.
As propene contains two similar groups (
(b)
Interpretation:
The compound 3-hexene exhibit geometric isomerism or not has to be given and two isomers has to be drawn and named for the given structure.
Concept Introduction:
The structure of the compound is given by its systematic name.
To give the structure from the name of the compound, the root name has to be identified. The root name indicates the number of carbon atoms present in the longest chain.
Then the functional group (suffix) has to be identified. It indicates whether any functional groups are present in the compound, it also gives whether the compound is an alkane or alkene or alkyne.
The prefix of the name indicates the branched groups and their positions on the carbon chain.
The name of the compound is in the form
Prefix + Root + Suffix
The geometric isomers are said to be the isomers which shows different orientation of groups around a double bond. The geometric isomers are also known as cis-trans isomers.
When two similar or higher priority groups are attached to the carbon on same side, it is said to be cis-isomer.
When two similar or higher priority groups are attached to the carbon on opposite sides, it is said to be trans-isomer.
To exhibit the geometric isomerism, a molecule should have double bonded carbon atoms (
(b)
Explanation of Solution
The given compound is 3-hexene.
The hexene contains six carbon atoms in chain. As the suffix is –ene, it belongs to alkene group and contains a double bond. The position of the double bond in 3-hexene is in between third and fourth carbon atoms. The structure of the 3-hexene is given as
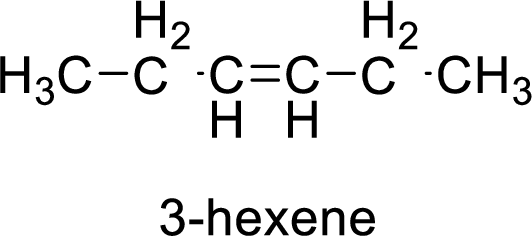
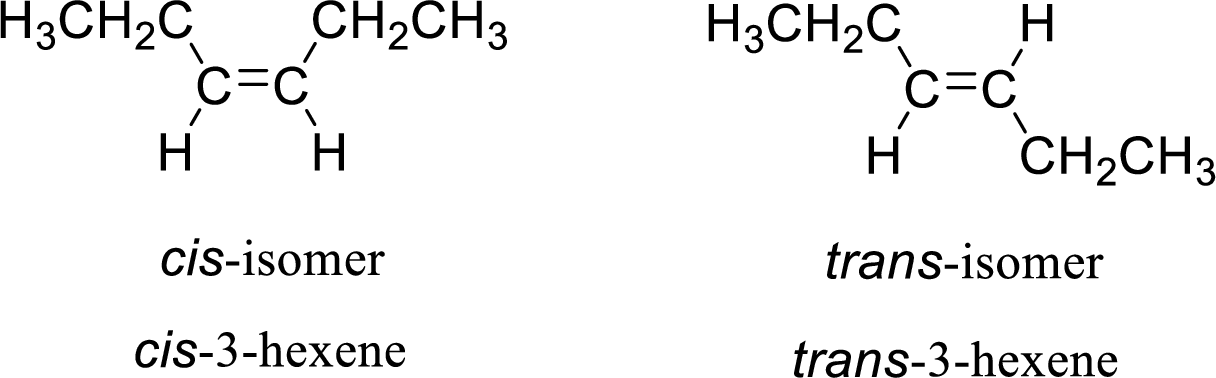
The given compound is 3-hexene. The given compound contains a double bond and each carbon in the double bond is bonded to two different groups. Hence, the given compound exhibits geometric isomerism.
The geometric isomers are cis-isomer and trans-isomer. In cis-isomer, similar or higher priority groups are attached to the carbon on same side and in trans-isomer, similar or higher priority groups are attached to the carbon on opposite sides.
The groups present on either sides of the double bond are given as higher priority groups (
(c)
Interpretation:
The compound 1,1-dichloroethene exhibit geometric isomerism or not has to be given and two isomers has to be drawn and named for the given structure.
Concept Introduction:
The structure of the compound is given by its systematic name.
To give the structure from the name of the compound, the root name has to be identified. The root name indicates the number of carbon atoms present in the longest chain.
Then the functional group (suffix) has to be identified. It indicates whether any functional groups are present in the compound, it also gives whether the compound is an alkane or alkene or alkyne.
The prefix of the name indicates the branched groups and their positions on the carbon chain.
The name of the compound is in the form
Prefix + Root + Suffix
The geometric isomers are said to be the isomers which shows different orientation of groups around a double bond. The geometric isomers are also known as cis-trans isomers.
When two similar or higher priority groups are attached to the carbon on same side, it is said to be cis-isomer.
When two similar or higher priority groups are attached to the carbon on opposite sides, it is said to be trans-isomer.
To exhibit the geometric isomerism, a molecule should have double bonded carbon atoms (
(c)
Explanation of Solution
The given compound is 1,1-dichloroethene.
The ethene contains two carbon atoms in chain. As the suffix is –ene, it belongs to alkene group and contains a double bond. The position of the double bond in ethene is in between first and second carbon atoms. Two chloro groups (
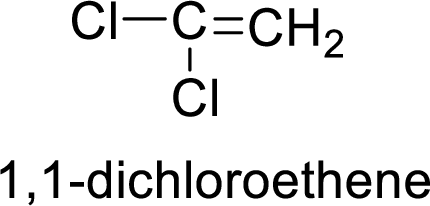
The given compound is 1,1-dichloroethene. The given compound contains a double bond and each carbon in the double bond is bonded to two groups.
The geometric isomers are cis-isomer and trans-isomer. In cis-isomer, similar groups are attached to the carbon on same side and in trans-isomer, similar groups are attached to the carbon on opposite sides.
As 1,1-dichloroethene contains two similar groups (
(d)
Interpretation:
The compound 1,2-dichloroethene exhibit geometric isomerism or not has to be given and two isomers has to be drawn and named for the given structure.
Concept Introduction:
The structure of the compound is given by its systematic name.
To give the structure from the name of the compound, the root name has to be identified. The root name indicates the number of carbon atoms present in the longest chain.
Then the functional group (suffix) has to be identified. It indicates whether any functional groups are present in the compound, it also gives whether the compound is an alkane or alkene or alkyne.
The prefix of the name indicates the branched groups and their positions on the carbon chain.
The name of the compound is in the form
Prefix + Root + Suffix
The geometric isomers are said to be the isomers which shows different orientation of groups around a double bond. The geometric isomers are also known as cis-trans isomers.
When two similar or higher priority groups are attached to the carbon on same side, it is said to be cis-isomer.
When two similar or higher priority groups are attached to the carbon on opposite sides, it is said to be trans-isomer.
To exhibit the geometric isomerism, a molecule should have double bonded carbon atoms (
(d)
Explanation of Solution
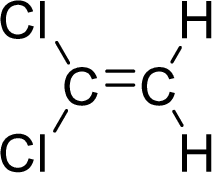
The given compound is 1,2-dichloroethene.
The ethene contains two carbon atoms in chain. As the suffix is –ene, it belongs to alkene group and contains a double bond. The position of the double bond in ethene is in between first and second carbon atoms. Two chloro groups (
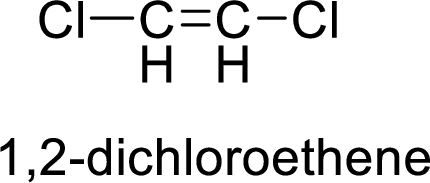
The given compound is 1,2-dichloroethene. The given compound contains a double bond and each carbon in the double bond is bonded to two different groups. Hence, the given compound exhibits geometric isomerism.
The geometric isomers are cis-isomer and trans-isomer. In cis-isomer, similar or higher priority groups are attached to the carbon on same side and in trans-isomer, similar or higher priority groups are attached to the carbon on opposite sides.
The groups present on either sides of the double bond are given as higher priority groups (
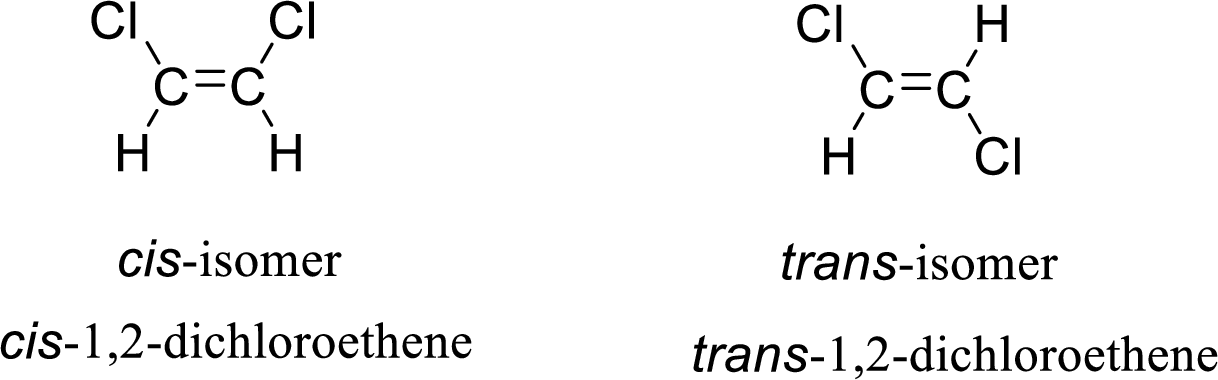
Want to see more full solutions like this?
Chapter 15 Solutions
MCGRAW: CHEMISTRY THE MOLECULAR NATURE
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





