
Concept explainers
(a)
Interpretation:
The hydrolysis of a 100.00-g sample of peptide forms the given individual amounts of amino acids (molar mass in g/mol are given in parentheses).
Why the total mass of amino acids exceeds the mass of peptide has to be given.
(a)
Explanation of Solution
The peptides are formed by the amino acid units. Amino acids combines together to form the amide bonds of peptides. The amide bond is formed by the loss of water molecule from
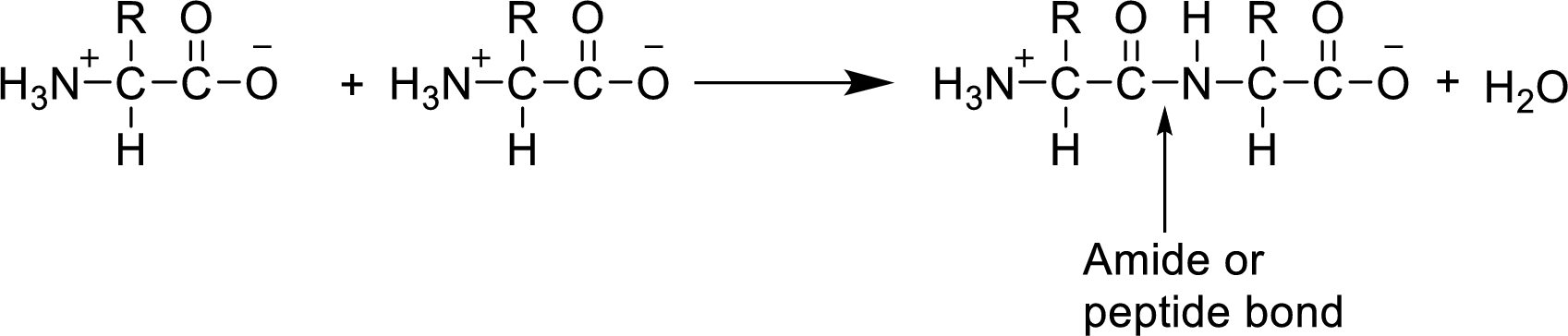
As the loss of water molecule occurs in the formation of peptide, the mass of peptide is less than that of total mass of amino acids.
(b)
Interpretation:
The hydrolysis of a 100.00-g sample of peptide forms the given individual amounts of amino acids (molar mass in g/mol are given in parentheses).
The relative numbers of amino acids in the peptide has to be given.
Concept Introduction:
The relative numbers can be calculated by converting the mass in to moles and by dividing the moles with smallest number.
(b)
Explanation of Solution
The given amounts of amino acids are
The mass of amino acids is converted to moles by using the equation
Glycine:
Alanine:
Valine:
Proline:
Serine:
Arginine:
The moles obtained can be divided by the smallest number to get the relative number.
The smallest number is
The relative numbers of amino acids are calculated.
(c)
Interpretation:
The hydrolysis of a 100.00-g sample of peptide forms the given individual amounts of amino acids (molar mass in g/mol are given in parentheses).
The minimum molar mass of the peptide has to be given.
Concept Introduction:
The minimum molar mass of peptide can be calculated by adding the products of moles of each amino acid with their molar mass to get the amount of amino acids and the water molecules mass is eliminated from the mass of amino acids to get the molar mass of peptide.
(c)
Explanation of Solution
The minimum molar mass of the peptide is calculated as
The minimum molar mass of peptide bond is calculated by subtracting the mass of water molecules from the mass of amino acids.
The minimum mass of peptide is
Want to see more full solutions like this?
Chapter 15 Solutions
Loose Leaf for Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





