
Concept explainers
(a)
The value of Q for path abc.
(a)
Answer to Problem 11P
The value of Q for path abc is
Explanation of Solution
Given:
The given path is shown below.
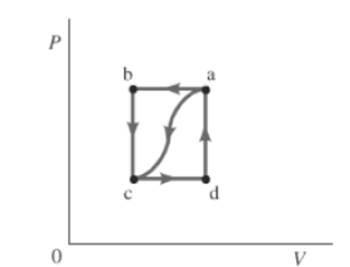
Also, the work done by the gas, from a to c is
Heat added to the gas is
Work done along path abc is
Formula used:
According to first law of
Where,
U is change in internal energy.
Q is heat added.
W is work done by the system.
Calculation:
Consider the given graph.
For path ac ,
Also, change in internal energy for closed path is zero. So,
For path abc ,
Conclusion:
Therefore, Q for path abc is
(b)
The value of W for path cda.
(b)
Answer to Problem 11P
The value of W for path cda is 24 J.
Explanation of Solution
Given:
The given path is shown below.
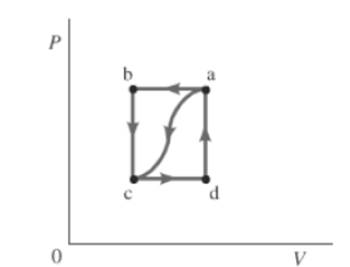
Also, the work done by the gas, from a to c is
Heat added to the gas is
Work done along path abc is
Formula used:
The work done is equal to the product of pressure and the change in the volume.
It is given as,
Where,
W is work done
P is pressure
Calculation:
Work done along path cda is equal to work done along path cd. There will be no work done across path da, because volume is constant. So,
Similarly, work done along path abc is equal to work done along path ab . There will be no work done across path bc , because volume is constant. So,
Conclusion:
Therefore, the value of W for path cda is 24 J.
(c)
The value of Q for path cda.
(c)
Answer to Problem 11P
The value of Q for path cda is 52 J.
Explanation of Solution
Given:
The given path is shown below.
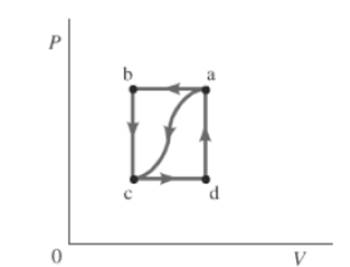
Also, the work done by the gas, from a to c is
Heat added to the gas is
Work done along path abc is
Formula used:
According to first law of thermodynamics,
Where,
U is change in internal energy.
Q is heat added.
W is work done by the system.
Calculation:
From part (b),
The value of W for cda is 24 J.
The change in internal energy for closed path is 0. So,
So for path cda ,
Conclusion:
Therefore, the value of Q for path cda is 52 J.
(d)
The value of
(d)
Answer to Problem 11P
The change in internal energy of the gas is 28 J.
Explanation of Solution
Given:
The given path is shown below.
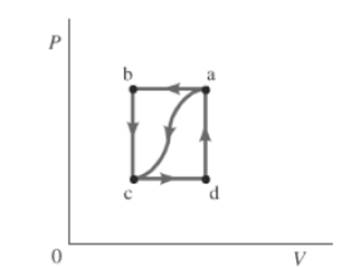
Also, the work done by the gas, from a to c is
Heat added to the gas is
Work done along path abc is
Formula used:
According to first law of thermodynamics,
Where,
U is change in internal energy.
Q is heat added.
W is work done by the system.
Calculation:
Consider the given graph.
For path ac ,
Conclusion:
Therefore, the change in internal energy of the gas is 28 J.
(e)
The value of Q for path da.
(e)
Answer to Problem 11P
The value of Q for path da is 23 J.
Explanation of Solution
Given:
The given path is shown below.
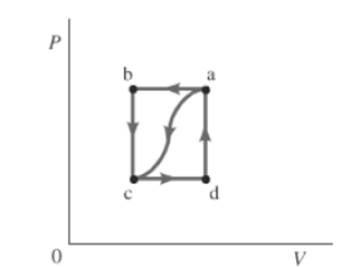
Also, the work done by the gas, from a to c is
Heat added to the gas is
Work done along path abc is
Formula used:
According to first law of thermodynamics,
Where,
U is change in internal energy.
Q is heat added.
W is work done by the system.
Calculation:
Consider the given graph.
The change in internal energy for closed path is 0. So,
So for path da,
There will be no work done across path da, because volume is constant. So,
Conclusion:
Therefore, the value of Q for path da is 23 J.
Chapter 15 Solutions
Physics: Principles with Applications
Additional Science Textbook Solutions
University Physics Volume 1
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Tutorials in Introductory Physics
Life in the Universe (4th Edition)
College Physics: A Strategic Approach (3rd Edition)
University Physics (14th Edition)
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





