
Concept explainers
Identify each structural formula as belonging to an
- (a) CH3CH2COOH
- (b)
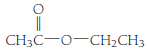
- (c) CH3CH2 CH2OH
- (d) CH3CH2NH2
- (e) CF3CF3
- (f)
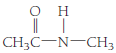
(a)
Answer to Problem 16E
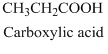
Explanation of Solution
The structural formula belongs to carboxylic acid family as it has
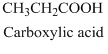
The structural formula of the compound has three carbon atoms longest chain which also includes
Conclusion:
Therefore, structurla formula belongs to carboxylic acid family. The structural formula of the compound is,
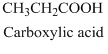
(b)
Answer to Problem 16E
Explanation of Solution
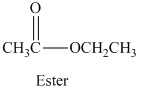
Esters are the organic compounds which are formed by the reaction of alcohols and carboxylic acid reaction, this reaction is also known as esterification reaction. When an alcohol reacts with a carboxylic acid the nucleophilic substitution takes place. The reaction can be written as,
The general molecular formula to identify an ester is
The structural formula of the compound is,

Conclusion:
Therefore structurla formula belongs to an ester family. The structural formula of the compound is,

(c)
Answer to Problem 16E
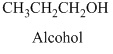
Explanation of Solution
Alcohols: The hydrocarbons in which hydrogen atom is replaced by hydroxy
Methanol is the first member of alcohol family it has
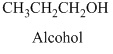
Therefore, this compound belongs to an alcohol family of organic compounds.
Conclusion:
Therefore structurla formula belongs to an alcohol family. The structural formula of the compound is
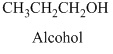
(d)
Answer to Problem 16E
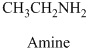
Explanation of Solution
A hydrocarbon in which hydrogen atom is replaced by
Here
Therefore, this given structural formula belongs to an amine family. The structure of the compound is,
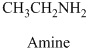
Conclusion:
The structurla formula belongs to an amine family. The structural formula of the compound is
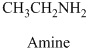
(e)
Answer to Problem 16E
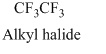
Explanation of Solution
Alkyl halides: When hydrogen atom is replaced by halogen atoms
Here,
The structural formula of the compound is,
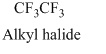
Conclusion:
Therefore, structurla formula belongs to an alkyl halides family. The structural formula of the compound is
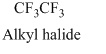
(f)
Answer to Problem 16E
The structural formula of the compound is
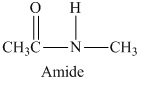 c
c
Explanation of Solution
Amides: These organic compounds are synthesized by the condensation reaction of carboxylic acids and amines. The functional group
A tertiary amine does not react with carboxylic acids; hence, no amide formation takes place. However, primary and secondary amines react with carboxylic acid and gives
The given structural formula belongs to the class of amides, which is formed by the reaction of acetic acid with methyl amine. The structural formula of the compound is,

Conclusion:
Therefore, structurla formula belongs to an amide family of organic compounds. The structural formula of the compound is

Want to see more full solutions like this?
Chapter 14 Solutions
INTRO TO PHYSICAL SCIENCE W/MINDTAP
Additional Science Textbook Solutions
Physical Universe
Schaum's Outline of College Physics, Twelfth Edition (Schaum's Outlines)
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
Physics for Scientists and Engineers with Modern Physics
Essential University Physics (3rd Edition)
The Cosmic Perspective Fundamentals (2nd Edition)
- Plants grow in many different shapes and sizes. Much of their shape depends on an internal structure that is composed of carbon-containing molecules such as cellulose and lignin. Plants that have a strong internal structure can grow larger than other plants because their structure can support their size. Plants obtain the majority of the carbon necessary for building these structural molecules from - O air O microorganisms O soil O water 10 11 12 13 14 15 16 17 18 19 20 近arrow_forwardGive the number of single, double, and triple bonds in the structural formula for CH3CH2C(O)CH3. Number of single bonds: Number of double bonds: Number of triple bonds:arrow_forwardWhy solvents are used to mimic the properties of biological membranes? Choose one of the following: and ered Butanol was converted into propanol. On-octanol is a kind of octanol.arrow_forward
- Which of the following statements are true with regards to alkanes, alkenes, and alkynes? Select all that apply. They are all hydrocarbons Alkanes have one or more double covalent bonds between carbon atoms Alkenes have one or more double covalent bonds between carbon atoms. Alkynes have one or more triple covalent bond between carbon atoms. O Alkenes have one or more double bond between either two carbon atoms, or a carbon and hydrogen atom.arrow_forward: P Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. H+ H+ + -OH ☑ Y Predict the organic products that form in the reaction below: Click and drag to start drawing a structure.arrow_forwardHow many products including stereoisomers are formed when 1,1,3,3- tetramethylcyclobutane undergoes monochlorination Determine the structure of the major monochlorination product of 1,1,3,3- tetramethylcyclobutane shown below using the reactivity order 5 : 3.5 : 1 for tertiary : secondary : primary hydrogens respectively.arrow_forward
- Write short note about molecular epitaxy?arrow_forwardWhat variables do you think are needed to determine the effectiveness of the antifreeze?arrow_forwardJustify the statement: Polymer molecular weight is expressed in terms of an average. Calculatethe number average and weight average molecular weights of polymer molecules with different degrees of polymerization such as 300, 550, 750 and 900 that are mixed in a molecular ratio 1: 2: 3: 4 in a sample of high polymer of styrene(C6H5 CH= CH2).arrow_forward
- The boiling point of an azeotropic mixture of water and ethanol is less than that of water and ethanol. The mixture shows (a) no deviation from Raoult’s Law. (b) positive deviation from Raoult’s Law. (c) negative deviation from Raoult’s Law. (d) that the solution is unsaturated.arrow_forwardHello please help answer this PH question? See attachementarrow_forward1.arrow_forward
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


