
(a)
Interpretation:
The acid-catalyzed hydrolysis of propyl propanoate has to be written.
Concept Introduction:
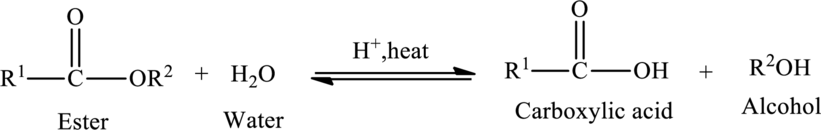
Hydrolysis of Ester:
The reaction in which a bond is broken by the addition of water molecule is called as hydrolysis.
Hydrolysis of esters yields the
The general reaction of ester hydrolysis is shown below,
(b)
Interpretation:
The acid-catalyzed hydrolysis of butyl methanoate has to be written.
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The acid-catalyzed hydrolysis of ethyl methanoate has to be written.
Concept Introduction:
Refer to part (a).
(d)
Interpretation:
The acid-catalyzed hydrolysis of methyl pentanoate has to be written.
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY
- personality of each of them in terms of nucleophile vs. electrophile (some can be considered acids/bases but we are not looking at that here). Note you may have to use your growing intuition to figure out the personality of one of the molecules below but I believe in you! Rationalize it out based on what we have called strong versus weak electrophiles in past mechanisms. Consider using the memes below to help guide your understanding! A OH O B CH3 C Molecule A: [Select] Molecule B: [Select] Molecule C: [Select] Molecule D: [Select] > H D OHarrow_forward4) Which oxygen atom in the structure below is most basic / nucleophilic? Please explain by discussing the electron density around each oxygen atom. Show at least three resonance structures for the compound. оогоarrow_forwardCan you show me this problem. Turn them into lewis dot structures for me please and then answer the question because I cant seem to comprehend it/ The diagrams on the picture look too small I guess.arrow_forward
- The fire releases 2.80 x 107 Joules of heat energy for each liter of oil burned. The water starts out at 24.5 °C, raising the water's temperature up to 100 °C, and then raises the temperature of the resulting steam up to 325 °C. How many liters of water will be needed to absorb the heat from the fire in this way, for each 1.0 liter of crude oil burned? 4186 J/(kg°C) = heat of water 2020 J/(kg°C) = heat of steam 2,256,000 (i.e. 2.256 x 106) J/kg = latent heat of vaporization for water (at the boiling point of 100 °C).arrow_forward6 Which of the following are likely to be significant resonance structures of a resonance hybrid? Draw another resonance structure for each of the compounds you select as being a resonance form. (A Br: Br: A B C D Earrow_forwardWrite the systematic (IUPAC) name for the following organic molecules. Note for advanced students: you do not need to include any E or Z prefixes in your names. Br structure Br Br Oweuarrow_forward
- Conservation of mass was discussed in the background. Describe how conservation of mass (actual, not theoretical) could be checked in the experiment performed.arrow_forwardWhat impact would adding twice as much Na2CO3 than required for stoichiometric quantities have on the quantity of product produced? Initial results attachedarrow_forwardGiven that a theoretical yield for isolating Calcium Carbonate in this experiment would be 100%. From that information and based on the results you obtained in this experiment, describe your success in the recovery of calcium carbonate and suggest two possible sources of error that would have caused you to not obtain 100% yield. Results are attached form experimentarrow_forward
- 5) Calculate the flux of oxygen between the ocean and the atmosphere(2 pts), given that: (from Box 5.1, pg. 88 of your text): Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturated What is flux if the temperature is 10°C ? (2 pts) (Hint: use the same density in your calculations). Why do your calculated values make sense (or not) based on what you know about the relationship between gas solubility and temperature (1 pt)?arrow_forwardFind a molecular formula for these unknownsarrow_forward(ME EX2) Prblms 8-11 Can you please explain problems 8 -11 to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





