
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134421353
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.54UTC
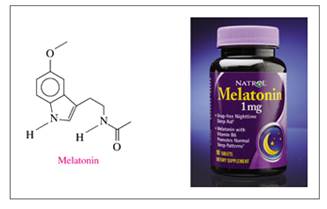
Melatonin is a naturally occurring compound in plants and animals, where it regulates the biological time clock. Melatonin is sometimes used to counteract jet lag. Identify the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used Ai solution
Don't used Ai solution
Please correct answer and don't used hand raiting
Chapter 14 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 14.1 - What carboxylic acid is responsible for the pain...Ch. 14.1 - What carboxylic acid is found in vinegar?Ch. 14.1 - Prob. 14.3PPCh. 14.1 - Prob. 14.4PPCh. 14.1 - Draw the condensed structural formulas for a and b...Ch. 14.1 - Draw the condensed structural formulas for a and b...Ch. 14.2 - Identify the compound in each group that is most...Ch. 14.2 - Prob. 14.8PPCh. 14.2 - Prob. 14.9PPCh. 14.2 - Prob. 14.10PP
Ch. 14.2 - Prob. 14.11PPCh. 14.2 - Prob. 14.12PPCh. 14.2 - Prob. 14.13PPCh. 14.2 - Prob. 14.14PPCh. 14.3 - Prob. 14.15PPCh. 14.3 - Prob. 14.16PPCh. 14.3 - Prob. 14.17PPCh. 14.3 - Prob. 14.18PPCh. 14.3 - Prob. 14.19PPCh. 14.3 - Prob. 14.20PPCh. 14.3 - Prob. 14.21PPCh. 14.3 - Prob. 14.22PPCh. 14.3 - Prob. 14.23PPCh. 14.3 - Prob. 14.24PPCh. 14.4 - What are the products of the acid hydrolysis of an...Ch. 14.4 - Prob. 14.26PPCh. 14.4 - Prob. 14.27PPCh. 14.4 - Prob. 14.28PPCh. 14.5 - Prob. 14.29PPCh. 14.5 - Prob. 14.30PPCh. 14.5 - Prob. 14.31PPCh. 14.5 - Prob. 14.32PPCh. 14.5 - Prob. 14.33PPCh. 14.5 - Prob. 14.34PPCh. 14.5 - Prob. 14.35PPCh. 14.5 - Prob. 14.36PPCh. 14.5 - Prob. 14.37PPCh. 14.5 - Prob. 14.38PPCh. 14.6 - Prob. 14.39PPCh. 14.6 - Prob. 14.40PPCh. 14.6 - Prob. 14.41PPCh. 14.6 - Prob. 14.42PPCh. 14.6 - Prob. 14.43PPCh. 14.6 - Prob. 14.44PPCh. 14.6 - Draw the condensed structural or line-angle...Ch. 14.6 - Draw the condensed structural or line-angle...Ch. 14.6 - a. Identify the functional groups in dicyclanil....Ch. 14.6 - a. Identify the functional groups in enrofloxacin....Ch. 14 - Prob. 14.49UTCCh. 14 - Prob. 14.50UTCCh. 14 - The ester methyl butanoate has the odor and flavor...Ch. 14 - Prob. 14.52UTCCh. 14 - Phenylephrine is the active ingredient in some...Ch. 14 - Melatonin is a naturally occurring compound in...Ch. 14 - Prob. 14.55UTCCh. 14 - Prob. 14.56UTCCh. 14 - Prob. 14.57APPCh. 14 - 14.58 Write the IUPAC and common names, if any,...Ch. 14 - Prob. 14.59APPCh. 14 - Prob. 14.60APPCh. 14 - Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.62APPCh. 14 - Prob. 14.63APPCh. 14 - 14.64 Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.65APPCh. 14 - 14.66 Write the common name and classify each of...Ch. 14 - Prob. 14.67APPCh. 14 - Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.69APPCh. 14 - Prob. 14.70APPCh. 14 - Write the IUPAC name for each of the following:...Ch. 14 - Prob. 14.72APPCh. 14 - Prob. 14.73APPCh. 14 - Prob. 14.74APPCh. 14 - Prob. 14.75APPCh. 14 - Toradol is used in dentistry to relieve pain....Ch. 14 - Prob. 14.77CPCh. 14 - Draw the line-angle formula and write the IUPAC...Ch. 14 - Prob. 14.79CPCh. 14 - Prob. 14.80CPCh. 14 - Prob. 14.81CPCh. 14 - Prob. 14.82CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY