
Concept explainers
(a)
Interpretation:
The product on reaction of 1-hexyne with HBr is to be stated.
Concept introduction:
The
Answer to Problem 14.26AP
The product on reaction of 1-hexyne with HBr is shown below.
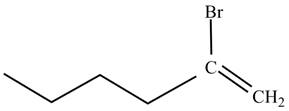
Explanation of Solution
The reaction of 1-hexyne with HBr is shown below.
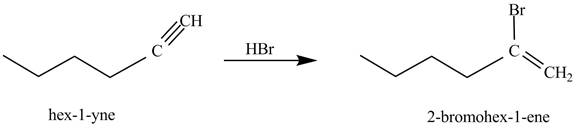
Figure 1
In the above reaction, hexyne reacts with HBr to form 2-bromohex-1-ene. Hexyne is an unsaturated molecule consisting of a triple bond. It reacts with ![]() to form a haloalkene. Therefore, the product on reaction of 1-hexyne with HBr is shown below.
to form a haloalkene. Therefore, the product on reaction of 1-hexyne with HBr is shown below.
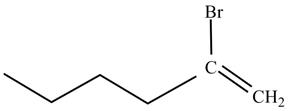
Figure 2
The product on reaction of 1-hexyne with HBr is shown in Figure 2.
(b)
Interpretation:
The product on reaction of 1-hexyne with H2,Pd/C catalyst is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of 1-hexyne with H2,Pd/C catalyst is shown below.
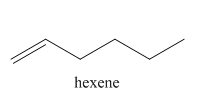
Explanation of Solution
The reaction of 1-hexyne with H2,Pd/C catalyst is shown below.
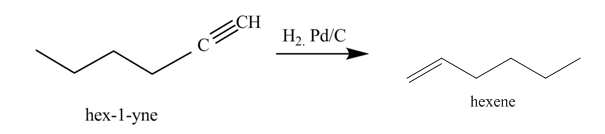
Figure 3
In the above reaction, hexyne reacts with hydrogen to form hexene. Hexyne is an unsaturated molecule consisting of a triple bond. It reacts hydrogen in presence of Pd/C to give

Figure 4
The product on reaction of 1-hexyne with H2,Pd/C catalyst is shown in Figure 4.
(c)
Interpretation:
The product on reaction of 1-hexyne with the H2,Pd/C, Lindlar’s catalyst is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of 1-hexyne with the H2,Pd/C, Lindlar’s catalyst is shown below.
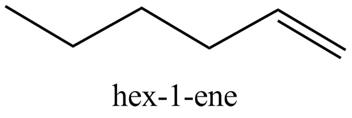
Explanation of Solution
The reaction of 1-hexyne with the H2,Pd/C, Lindlar’s catalyst is shown below.
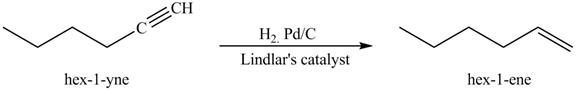
Figure 5
In the above reaction, hexyne reacts with H2,Pd/C in presence of Lindlar’s catalyst to form an alkene. Due to the presence of Lindlar’s catalyst the hydrogenation of alkyne stops at alkene and does not proceed further to give
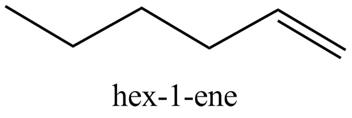
Figure 6
The product on reaction of 1-hexyne with the H2,Pd/C, Lindlar’s catalyst is shown in Figure 6.
(d)
Interpretation:
The product on reaction of the product formed in part (c) with O3 and then with (CH3)2S is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of the product formed in part (c) with O3 and then with (CH3)2S is shown below.
Explanation of Solution
The product formed in part (c) is shown below.
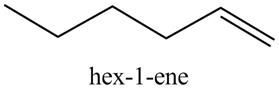
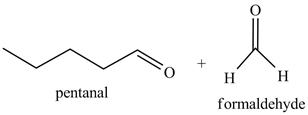
Figure 7
The reaction of the product formed in part (c) with O3 and then with (CH3)2S is shown below.
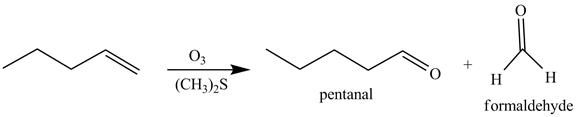
Figure 8
The above reaction is known as ozonlysis reaction. In the above reaction, a double bond is cleaved and oxidized to give two products. Reaction of hexene with O3 followed by (CH3)2S forms pentanal and formaldehyde. Therefore, the products formed on reaction of the product formed in part (c) with O3 and then with (CH3)2S are shown below.

Figure 9
The products on reaction of reaction of the product formed in part (c) with O3 and then with (CH3)2S are shown in Figure 9.
(e)
Interpretation:
The product on reaction of the product formed in part (c) with BH3 in THF, then H2O2/−OH is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of the product formed in part (c) with BH3 in THF, then H2O2/−OH is shown below.
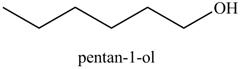
Explanation of Solution
The product formed in part (c) is shown below.

Figure 7
The reaction of above compound with BH3 in THF, then H2O2/−OH
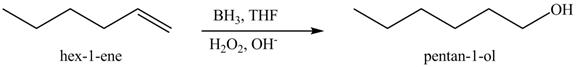
Figure 10
In the above reaction, hexene reacts with boron hydride to form organoborane which further reacts with peroxide to form an alcohol. The alcohol thus formed is by anti-markovnikov addition.

Figure 11
The product on reaction of the product formed in part (c) with BH3 in THF, then H2O2/−OH is shown in Figure 11.
(f)
Interpretation:
The product on reaction of the product formed in part (c) with Br2 is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of the product formed in part (c) with Br2 is shown below.
Explanation of Solution
The product formed in part (c) is shown below.
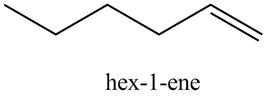
Figure 7
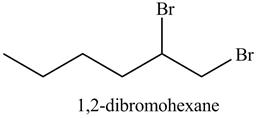
The reaction of above compound with Br2 is shown below.
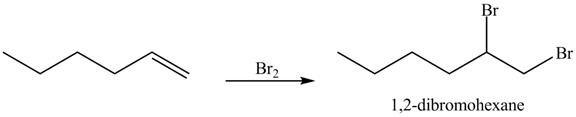
Figure 12
In the above reaction, hexene being unsaturated reacts with bromine molecule to form a dibromoalkane, that is 1, 2-dibromohexane. In the above reaction, unsaturated molecule is forming a saturated dihaloalkane on reaction with bromine molecule. Therefore, the product formed on reaction of product formed in (c) with bromine molecule is shown below.
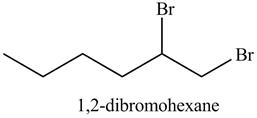
Figure 13
The product on reaction of the product formed in part (c) with Br2 is shown in Figure 13.
(g)
Interpretation:
The product on reaction of 1-hexyne with NaNH2 in liquid ammonia is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of 1-hexyne with NaNH2 in liquid ammonia is shown below.
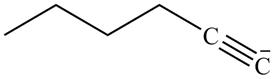
Explanation of Solution
The reaction of 1-hexyne with NaNH2 in liquid ammonia is shown below.
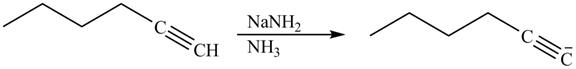
Figure 14
Alkynes react with sodamide (NaNH2) in presence of liquid ammonia to release an acidic proton and to form an anion of alkyne. Therefore, on reaction of 1-hexyne with NaNH2 in liquid ammonia releases the acidic proton to form an anion of hexyne. The product therefore formed is shown below.

Figure 15
The product on reaction of 1-hexyne with NaNH2 in liquid ammonia is shown in Figure 15.
(h)
Interpretation:
The product on reaction of the product formed in part (g) with CH3CH2I is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of the product formed in part (g) with CH3CH2I is shown below.
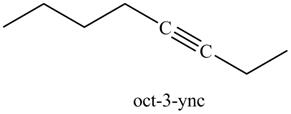
Explanation of Solution
The product formed in part (g) is shown below.
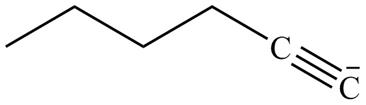
Figure 15
The reaction of above compound with CH3CH2I is shown below.
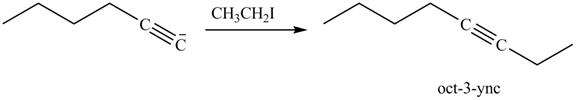
Figure 16
In the above reaction, the anion of alkyne obtained reacts with iodoethane to form an alkyne of eight carbons. It is non-terminal alkyne, that is, triple bond is not situated at the terminal end. The product formed is shown below.
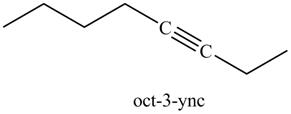
Figure 17
The product on reaction of the product formed in part (g) with CH3CH2I is shown in Figure 17.
(i)
Interpretation:
The product on reaction of 1-hexyne with Hg2+, H2SO4, H2O is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of 1-hexyne with Hg2+, H2SO4, H2O is shown below.
Explanation of Solution
The reaction of 1-hexyne with Hg2+, H2SO4, H2O is shown below.
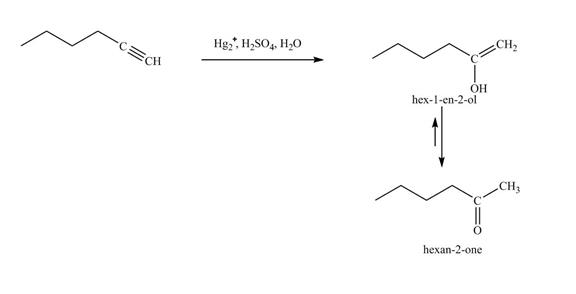
Figure 18
In the above reaction, hexyne reacts with Hg2+, H2SO4, H2O to form a

Figure 19
The product on reaction of 1-hexyne with Hg2+, H2SO4, H2O is shown in Figure 19.
(j)
Interpretation:
The product on reaction of 1-hexyne with disiamyl borane, H2O2/−OH is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of 1-hexyne with disiamyl borane, H2O2/−OH is shown below.
Explanation of Solution
The reaction of 1-hexyne with disiamyl borane, H2O2/−OH is shown below.
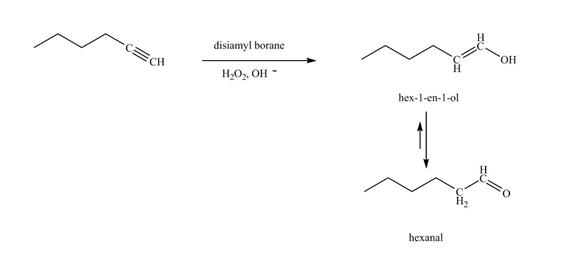
Figure 20
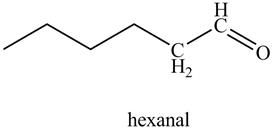
In the above reaction, hexyne reacts with disiamyl borane to form an organoborane. This reaction is also known as hydroboration-oxidation reaction. The organo-borane gives an enol which tautomerism to form an
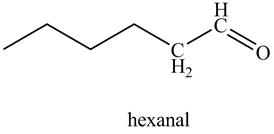
Figure 21
The product on reaction of 1-hexyne with disiamyl borane, H2O2/−OH is shown in Figure 21.
(k)
Interpretation:
The product on reaction of 1-hexyne with CH3CH2MgBr in ether is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
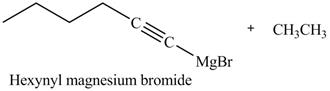
The products formed on reaction of 1-hexyne with CH3CH2MgBr in ether are shown below.
Explanation of Solution
The reaction of 1-hexyne with CH3CH2MgBr in ether is shown below.
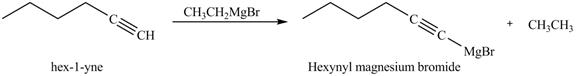
Figure 22
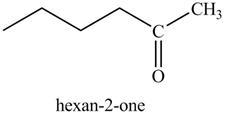
In the above reaction, CH3CH2MgBr is Grignard reagent that reacts with alkyne to form an alkane, that is ethane and another Grignard reagent. Hexyne on reaction with Grignard reagent releases the cationic terminal hydrogen which goes on to react with the negative part of Grignard reagent and forms an alkane. The anionic part of alkyne and positive part of Grignard reagent react to form another Grignard reagent. The products therefore obtained are shown below.

Figure 23
The products formed on reaction of 1-hexyne with CH3CH2MgBr are shown in Figure 23.
(l)
Interpretation:
The product on reaction of the product formed in part (k) with ethylene oxide and H3O+ is to be stated.
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is CnH2n−2. The name of the alkyne compounds ends with suffix −yne. Hydrocarbons can also exist in ring like structures.
Answer to Problem 14.26AP
The product on reaction of the product formed in part (k) with ethylene oxide and H3O+ is shown below.
Explanation of Solution
The product formed in part (k) is shown below.
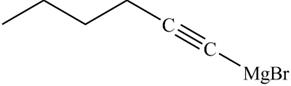
Figure 23
The reaction of above compound with ethylene oxide and H3O+ is shown below.
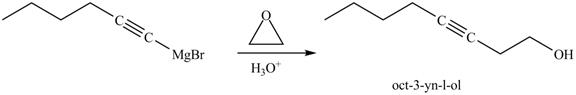
Figure 24
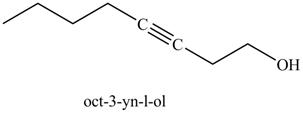
In the above reaction, the Grignard reagent obtained in part (k) reacts with ethylene oxide followed by hydrolysis to form an alcohol with a triple bond in between the chain. The product formed on reaction of product formed in part (k) with ethylene oxide and H3O+ is shown below.
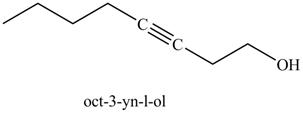
Figure 25
The product on reaction of the product formed in part (k) with ethylene oxide and H3O+ is in Figure 25.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry
- If a high molecular weight linear polyethylene is chlorinated by inducing the substitution of chlorine atoms by hydrogen, if 5% of all hydrogen atoms are replaced, what approximate percentage of chlorine by weight would the product have?arrow_forwardO Macmillan Learning Chemistry: Fundamentals and Principles Davidson presented by Macmillan Learning Poly(ethylene terephthalate), known as PET or industrially as Dacron, is a polyester synthesized through a condensation reaction between two bifunctional monomers. The monomers, ethylene glycol and terepthalic acid, are given. Add bonds and remove atoms as necessary to show the structure of a two repeat unit portion of a longer polymer chain of PET. You may need to zoom out to see the complete structure of all four monomer units. Select Draw / || | C H 0 3 © Templates More ° ° ° || C CC - OH HO OH HOC - C Erase CC OH HO C C 〃 C H₂ Q2Qarrow_forwardc) + H₂Oarrow_forward
- 으 b) + BF. 3 H2Oarrow_forwardQ4: Draw the product of each Lewis acid-bas reaction. Label the electrophile and nucleophile. b) S + AICI 3 + BF 3arrow_forwardQ1 - What type(s) of bonding would be expected for each of the following materials: solid xenon, calcium fluoride (CaF2), bronze, cadmium telluride (CdTe), rubber, and tungsten? Material solid xenon CaF2 bronze CdTe rubber tungsten Type(s) of bonding Q2- If the atomic radius of lead is 0.175 nm, calculate the volume of its unit cell in cubic meters.arrow_forward
- Determine the atomic packing factor of quartz, knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are 0.038 and 0.117 nm.arrow_forwardUse the following data for an unknown gas at 300 K to determine the molecular mass of the gas.arrow_forward2. Provide a complete retrosynthetic analysis and a complete forward synthetic scheme to make the following target molecule from the given starting material. You may use any other reagents necessary. Brarrow_forward
- 146. Use the following data for NH3(g) at 273 K to determine B2p (T) at 273 K. P (bar) 0.10 0.20 0.30 0.40 0.50 0.60 (Z -1)/10-4 1.519 3.038 4.557 6.071 7.583 9.002 0.70 10.551arrow_forward110. Compare the pressures given by (a) the ideal gas law, (b) the van der Waals equation, and (c) the Redlic-Kwong equation for propane at 400 K and p = 10.62 mol dm³. The van der Waals parameters for propane are a = 9.3919 dm6 bar mol-2 and b = 0.090494 dm³ mol−1. The Redlich-Kwong parameters are A = 183.02 dm bar mol-2 and B = 0.062723 dm³ mol-1. The experimental value is 400 bar.arrow_forwardResearch in surface science is carried out using stainless steel ultra-high vacuum chambers with pressures as low as 10-12 torr. How many molecules are there in a 1.00 cm3 volume at this pressure and at a temperature of 300 K? For comparison, calculate the number of molecules in a 1.00 cm3 volume at atmospheric pressure and room temperature. In outer space the pressure is approximately 1.3 x 10-11 Pa and the temperature is approximately 2.7 K (determined using the blackbody radiation of the universe). How many molecules would you expect find in 1.00 cm3 of outer space?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





