
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.7, Problem 13.5P
Following are two constitutional isomers with the molecular formula C4H8O2.
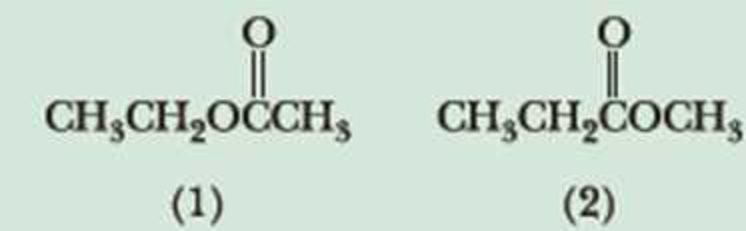
- (a) Predict the number of signals in the 1H-NMR spectrum of each isomer.
- (b) Predict the ratio of areas of the signals in each spectrum.
- (c) Show how you can distinguish between these isomers on the basis of chemical shift.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Indicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are false
(f) SO:
Best Lewis Structure
3
e group geometry:_
shape/molecular geometry:,
(g) CF2CF2
Best Lewis Structure
polarity:
e group arrangement:_
shape/molecular geometry:
(h) (NH4)2SO4
Best Lewis Structure
polarity:
e group arrangement:
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
Sketch (with angles):
1.
Problem Set 3b
Chem 141
For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing
bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the
molecule is polar or non-polar (iv)
(a) SeF4
Best Lewis Structure
e group arrangement:_
shape/molecular geometry:
polarity:
(b) AsOBr3
Best Lewis Structure
e group arrangement:_
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
Chapter 13 Solutions
Organic Chemistry
Ch. 13.2 - Calculate the ratio of nuclei in the higher spin...Ch. 13.5 - State the number of sets of equivalent hydrogens...Ch. 13.5 - Each compound gives only one signal in its 1H-NMR...Ch. 13.6 - The line of integration of the two signals in the...Ch. 13.7 - Following are two constitutional isomers with the...Ch. 13.8 - Following are pairs of constitutional isomers....Ch. 13.10 - Following is a 1H-NMR spectrum of 2-butanol....Ch. 13.11 - Explain how to distinguish between the members of...Ch. 13 - Prob. 13.9PCh. 13 - Prob. 13.10P
Ch. 13 - Prob. 13.11PCh. 13 - Following are structural formulas for three...Ch. 13 - Following arc structural formulas for the cis...Ch. 13 - Prob. 13.14PCh. 13 - Following are three compounds with the molecular...Ch. 13 - Following are 1H-NMR spectra for compounds D, E,...Ch. 13 - Following are 1H-NMR spectra for compounds G, H,...Ch. 13 - Propose a structural formula for compound J,...Ch. 13 - Compound K, molecular formula C6H14O, readily...Ch. 13 - Compound M, molecular formula C5H10O, readily...Ch. 13 - Following is the 1H-NMR spectrum of compound O,...Ch. 13 - Treatment of compound P with BH3 followed by...Ch. 13 - The 1H-NMR spectrum of compound R, C6H14O,...Ch. 13 - Write structural formulas for the following...Ch. 13 - Prob. 13.25PCh. 13 - Ascaridole is a natural product that has been used...Ch. 13 - The 13C-NMR spectrum of 3-methyl-2-butanol shows...Ch. 13 - Prob. 13.28P
Additional Science Textbook Solutions
Find more solutions based on key concepts
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
Give the IUPAC name for each compound.
Organic Chemistry
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY