
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.7, Problem 13.5P
Following are two constitutional isomers with the molecular formula C4H8O2.
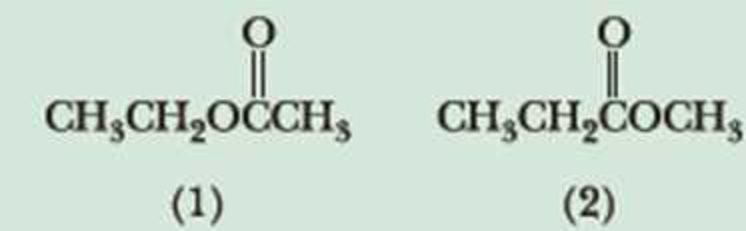
- (a) Predict the number of signals in the 1H-NMR spectrum of each isomer.
- (b) Predict the ratio of areas of the signals in each spectrum.
- (c) Show how you can distinguish between these isomers on the basis of chemical shift.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
(a) Draw all six isomers of formula C4H8 (including stereoisomers).(b) For each structure, show how many types of H would appear in the proton NMR spectrum.(c) For each structure, show how many types of C would appear in the 13C NMR spectrum.(d) If an unknown compound of formula C4H8 shows two types of H and three types of C, can you determine its structurefrom this information?
The 1H NMR spectrum of 1,2-dimethoxyethane (CH3OCH2CH2OCH3) recorded on a 300 MHz NMR spectrometer consists of signals at 1017 Hz and 1065 Hz downeld from TMS. (a) Calculate the chemical shift of each absorption. (b) At what frequency would each absorption occur if the spectrum were recorded on a 500 MHz NMR spectrometer?
(a) Draw all six isomers of formula C4H8 (including stereoisomers).(b) For each structure, show how many types of H would appear in the proton NMR spectrum.(c) For each structure, show how many types of C would appear in the 13C NMR spectrum.
Chapter 13 Solutions
Organic Chemistry
Ch. 13.2 - Calculate the ratio of nuclei in the higher spin...Ch. 13.5 - State the number of sets of equivalent hydrogens...Ch. 13.5 - Each compound gives only one signal in its 1H-NMR...Ch. 13.6 - The line of integration of the two signals in the...Ch. 13.7 - Following are two constitutional isomers with the...Ch. 13.8 - Following are pairs of constitutional isomers....Ch. 13.10 - Following is a 1H-NMR spectrum of 2-butanol....Ch. 13.11 - Explain how to distinguish between the members of...Ch. 13 - Prob. 13.9PCh. 13 - Prob. 13.10P
Ch. 13 - Prob. 13.11PCh. 13 - Following are structural formulas for three...Ch. 13 - Following arc structural formulas for the cis...Ch. 13 - Prob. 13.14PCh. 13 - Following are three compounds with the molecular...Ch. 13 - Following are 1H-NMR spectra for compounds D, E,...Ch. 13 - Following are 1H-NMR spectra for compounds G, H,...Ch. 13 - Propose a structural formula for compound J,...Ch. 13 - Compound K, molecular formula C6H14O, readily...Ch. 13 - Compound M, molecular formula C5H10O, readily...Ch. 13 - Following is the 1H-NMR spectrum of compound O,...Ch. 13 - Treatment of compound P with BH3 followed by...Ch. 13 - The 1H-NMR spectrum of compound R, C6H14O,...Ch. 13 - Write structural formulas for the following...Ch. 13 - Prob. 13.25PCh. 13 - Ascaridole is a natural product that has been used...Ch. 13 - The 13C-NMR spectrum of 3-methyl-2-butanol shows...Ch. 13 - Prob. 13.28P
Additional Science Textbook Solutions
Find more solutions based on key concepts
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living By Chemistry: First Edition Textbook
During the early part of the 20th century, sulfanilamide (an antibacterial drug) was only administered by injec...
Elementary Principles of Chemical Processes, Binder Ready Version
Characterize each of the following structures as aromatic, nonaromatic, or antiaromatic:
Answer: _____
Organic Chemistry As a Second Language: Second Semester Topics
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
22.102 Write the structures of the cis and tram isomers, if any, for the following compounds:
Chemistry: The Molecular Nature of Matter
Determine the number of protons, neutrons, and electrons in the following atoms: a. a hydrogen atom that has a ...
General, Organic, and Biological Chemistry (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the approximate chemical shifts of the protons in the following compounds. (a) benzene (b) cyclohexanearrow_forwardThe 1H NMR spectrum of 1,2-dimethoxyethane (CH3OCH2CH2OCH3) recorded on a 300 MHz NMR spectrometer consists of signals at 1017 Hz and 1065 Hz downfield from TMS.(a) Calculate the chemical shift of each absorption. (b) At what frequency would each absorption occur if the spectrum were recorded on a 500 MHz NMR spectrometer?arrow_forwardThe 'H NMR spectrum of 1,2-dimethoxyethane (CH;OCH,CH2OCH3) recorded on a 300 MHz NMR spectrometer consists of signals at 1017 Hz and 1065 Hz downfield from TMS. (a) Calculate the chemical shift of each absorption. (b) At what frequency would each absorption occur if the spectrum were recorded on a 500 MHz NMR spectrometer?arrow_forward
- 6) Complete the spectroscopy data tables for a compound with molecular formula C6H12O. Determine the structure of the compound. Any labile protons, if they exist, will not be present in this particular 1H NMR spectrum.arrow_forwardWhat is the molecular formula structure and its proton environment of these two spectrum?arrow_forwardWhich compound gives a signal in the 1H-NMR spectrum with a larger chemical shift, furan or cyclopentadiene? Explain.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY