
Concept explainers
A spring-loaded piston–cylinder device contains a mixture of gases whose pressure fractions are 25 percent Ne, 50 percent O2, and 25 percent N2. The piston diameter and spring are selected for this device such that the volume is 0.1 m3 when the pressure is 200 kPa and 1.0 m3 when the pressure is 1000 kPa. Initially, the gas is added to this device until the pressure is 200 kPa and the temperature is 10°C. The device is now heated until the pressure is 500 kPa. Calculate the total work and heat transfer for this process.
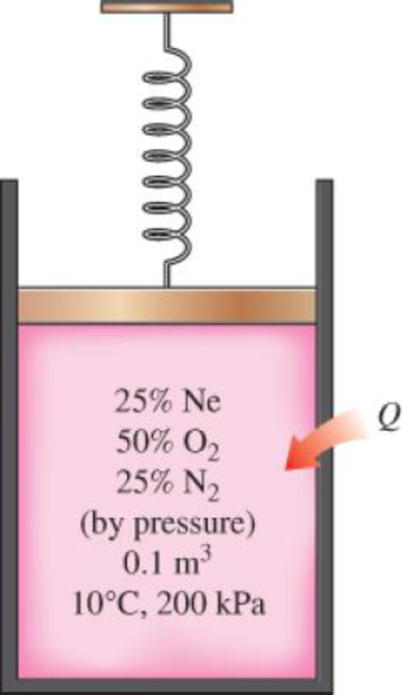
The total work done and heat transfer for the process.
Answer to Problem 94RP
The total work done for the process is
The heat transfer for the process is
Explanation of Solution
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the mass of
Write the expression to calculate the mass of
Write the expression to calculate the mass of
Write the expression to calculate the total mass of each component
Write the expression to calculate the mass fraction of
Write the expression to calculate the mass fraction of
Write the expression to calculate the mass fraction of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the total number of moles
Write the expression to calculate the apparent molecular weight of the mixture
Write the expression to calculate the constant volume specific heat of the mixture
Here, mole fraction of
Write the expression to calculate the apparent gas constant of the mixture
Here, universal gas constant is
Write the expression for the mass contained in the system
Write the expression to calculate the final temperature
Write the expression to calculate the work done during the process.
Conclusion:
From Table A-1, “Molar mass, gas constant, and critical point properties”, obtain the values of molar masses for
From Table A-2a, “Ideal-gas specific heats of various common gases”, obtain the following properties for
For
For
For
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
The pressure changes linearly with volume as shown is Figure (1).
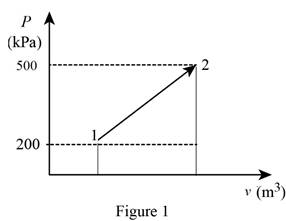
Using the data form Prob. 13–94 obtain the value of final volume by linear interpolation.
Write the straight line equation for two points.
Here, coordinates of the point 1 is
Substitute
The final volume
Substitute
Substitute
Thus, the total work done for the process is
Write a energy balance on the system.
Here, input energy transfer and output energy transfer is
The rate of change in energy of a system
For given system the energy balance Equation (XXII) is expressed as follows:
The rate of change in energy of a system
Substitute
Thus, the heat transfer for the process is
Want to see more full solutions like this?
Chapter 13 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- The dryness degree of the water in the saturated liquid-steam mixture at 90 ° C in a 5 liter container is 0.5. How many grams is the mass of water in this container?arrow_forwardA steel tank initially contains acetylene gas at 450kPag and 21°C. After 1.25kg of the gas is used the pressure and temperature of the gas inside the tank was found to be 180kPag and 20°C, respectively. What is the initial mass of the gas?arrow_forwardA rigid tank contains 2.0 kg of nitrogen at 100 K and ? = 0.50. Then, 0.5 kg ofnitrogen is removed from the tank through a valve. If the temperature remains constant throughout the process, determine the final state of the nitrogen inside the tank and the volume of nitrogen removed if the valve is located at(a) The top(b) The bottomarrow_forward
- Q Search A tank with a volume of 3.5 m³ is filled with carbon monoxide at 53°C and 170 kPa is connected through a valve to another tank containing 3.6 kg of carbon monoxide at 100°C and 300 kPa. Now the valve is opened, and the entire system. If the final temperature of the entire system is 18°C, determine the volume of the second tank and the final equilibrium pressure of the carbon monoxide.arrow_forwardWater is contained in a cylinder/piston arrangement fitted with lower stops. Initially, the cylinder contains a mixture of 0.75 kg of liquid and 0.25 Kg of vapor, and the pressure is 210 kPa. The water is heated slowly, and the piston, which initially rests on the stops, starts moving when the pressure inside reaches 600 kPa. Heating continues until the volume is 3 times the initial volume. Determine: (a) The initial and final temperatures. (b) The mass of the liquid when the piston first starts moving (c) The work done during this process Also, sketch the process on both the P-v diagram.arrow_forwardA medical ampoule containing 10 MPa, dextrose (liquid water) at 155(°c)and 0.5∙10-5 m3 in volume is placed in a 0.03 m3 volume cylinder. A vacuum is created in the cylinder and the capsule ruptures. Then the dextrose fills the cylinder and begins to evaporate. Calculate the final quality of the water-vapor mixture in the cylinder when it reaches the final equilibrium temperature of 35 oC. Also, calculate the heat transfer with the environment.arrow_forward
- A 4.081-kg steam-water mixture at 1.0 MPa is contained in an inflexible tank. Heat is added until the pressure rises to 3.5 MPa and the temperature to 400°C. Determine the heat added in kJ.arrow_forwardOn a hot summer day, you decide to make some iced tea. First, you brew 1.50 L of hot tea and leave it to steep until it has reached a temperature of Ttea = 75.0 ∘C. You then add 0.975 kg of ice taken from the freezer at a temperature of Tice = 0 ∘C. By the time the mix reaches equilibrium, all of the ice has melted. What is the final temperature Tf of the mixture? For the purposes of this problem, assume that the tea has the same thermodynamic properties as plain water. The specific heat of water is c = 4190 J/kg⋅∘C The heat of fusion of ice is Lf = 3.33×105 J/kg The density of the tea is ρ tea = 1.00 kg/Larrow_forwardA rigid tank contains 2kg of saturated water vapor mixture at temperature of 50 degrees C and quality of 70%. Electrical heating is used to convert this to superheated steam at 1.2MPa and 250 degrees C. If the heat transer from the tank during this process is 500kJ, what is the required electrical energy? What happens if the process continues ti 10 minutes, what is the required electrical power?arrow_forward
- A rigid container with a volume of 2000 liters contains 4 kg mixture of saturated water and steam at 120°C. The mixture is slowly heated until the liquid content is completely vaporized. Determine the following: a) quality of the vapor at the initial condition; and b) temperature of water after heating. From steam tables @t: = 120°C (vn = 1.0603x10' m'/kg, vạ1 = 891.9x10' m²/kg). HINT: If v is 508.9 x10 there are 140°C, if v = 495.6x10 there are 141°carrow_forwardA 3.586-kg steam-water mixture at 1.0 MPa is contained in an inflexible tank. Heat is added until the pressure rises to 3.5 MPa and the temperature to 400°C. Determine the heat added in kj. For the steam table, please refer to the green book entitled " Thermodynamic Properties of Water Including Vapor, Liquid, and Solid Phases"arrow_forward4. Carbon dioxide gas enters a pipe at 3 MPa and 500 K at a rate of 2 kg/s. CO2 is cooled at constant pressure as it flows in the pipe and the temperature of CO2 drops to 450 K at the exit. Determine the volume flow rate and the density of carbon dioxide at the inlet and the volume flow rate at the exit of the pipe using: (a) the ideal-gas equation and (b) the generalized compressibility chart. (c) Also, determine the error involved in the first case 3 МРа 500 K CO - 450 K 2 kg/sarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





