
Concept explainers
(a)
Interpretation:
The IUPAC name for the following alkene should be determined:
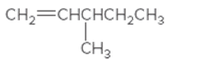
Concept Introduction:
(b)
Interpretation:
The IUPAC name for the following alkene should be determined:
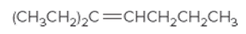
Concept Introduction:
Alkenes are named in the same way as alkanes, but the alkenes are identified by the suffix −ene, replaced instead of the ending of the name of the parent alkane. The longest carbon chain should be numbered in a way that gives the double bond the lower number. Then the compound should be named using the first number assigned to the double bond. The names of the substituents should be written first in the alphabetic order with their position on the chain.
(c)
Interpretation:
The IUPAC name for the following alkene should be determined:
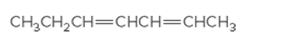
Concept Introduction:
Alkenes are named in the same way as alkanes, but the alkenes are identified by the suffix −ene, replaced instead of the ending of the name of the parent alkane. The longest carbon chain should be numbered in a way that gives the double bond the lower number. Then the compound should be named using the first number assigned to the double bond. The names of the substituents should be written first in the alphabetic order with their position on the chain.
(d)
Interpretation:
The IUPAC name for the following alkene should be determined:
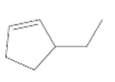
Concept Introduction:
In the nomenclature of cycloalkenes, the double bond is always located between C-1 and C-2. And 1 is not mentioned in the name. the ring is then numbered in a way that the first substituent getting the lowest number.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Connect 1-Semester Access Card for General, Organic, & Biological Chemistry
- I. Write the IUPAC name of each organic compound. Structure NH2-C-CH-C СН 2-О-СН2-СH3 ČI ČI 1. H3C-CH=CH-C-CH, 2. OH CH CHCH CC=CH CH CH 3.arrow_forwardGive the IUPAC name for each cycloalkane.arrow_forwardWhich structure below represents 4-methylpent-2-ene? a. CH3-CH2-CH2-CH2-C=CH2 O b. CH3-CH2-C=CH-CH3 O c. O d. CH2-CH3 CH3 CH3-CH-CH=CH-CH3 CH3 CH3-CH2-CH-CH=CH2 CH, CH3arrow_forward
- Give the IUPAC name for each compound. Part 1 of 3 H,CCH H С. CH3 -CH,CH,CH,CH, Part 2 of 3 Х б CCH, CH2- CH, H | С. H | - -CH2CH2CH2CH3 CH2CH2CH3 00 18 Ar Яarrow_forward9 and 10arrow_forwardStructure A Cis Isomer H₂C Trans Isomer Name of the Alkene H C=C CH₂CH₂ H [Choose ] [Choose ] Pentene Propene Hexene Structure A Structure B [Choose ] H₂C H C=C Structure B I CH₂CH₂arrow_forward
- Draw all constitutional isomers formed when each alkene is treated with NBS + hv. -CH3 CH c. CH2=C(CH2CH3)2 a. CH,CH=CHCH3 b.arrow_forwardtheir IUPAC names?arrow_forward4. Use the IUPAC Nomenclature System to name each of the following compounds: a. b. CH,CCH₂CH, 0 HCCHCH₂CH₂ CI O -C-C-CH₂ Br CHỊCH,CH,CH, CH,CCH₂CH₂CH CH, CI CH₂CHCH₂CH Br CH, Ô CH,CCH.CCH.CH.CH, OH 0 CH₂ CH,CCH₂CH.CH,CHCH,C-H H,CHCH.CH CH₂arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





