
Physics for Scientists and Engineers with Modern Physics
4th Edition
ISBN: 9780131495081
Author: Douglas C. Giancoli
Publisher: Addison-Wesley
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13.10, Problem 1GE
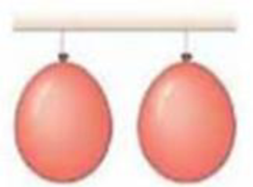
Return to Chapter-Opening Question 2, page 339, and answer it again now. Try to explain why you may have answered differently the first lime. Try it and sec.
Two balloons are tied and hang with their nearest edges about 3 cm apart. If you blow between the balloons (not at the balloons, but at the opening between them), what will happen?
- (a) Nothing.
- (b) The balloons will move closer together.
- (c) The balloons will move farther apart.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Solids and liquids resist being compressed. They are
not totally incompressible, but it takes large forces to
compress them even slightly.
Part A
If it is true that matter consists of atoms, what can you infer about the microscopic nature of solids and liquids from their
incompressibility?
Drag the terms on the left to the appropriate blanks on the right to complete the sentences.
can't
rarely
can
essentially
Reset Help
Review | Constants
The fact that liquids and solids
be compressed tells us that the
atoms/molecules of liquids and solids are
can't in contact with each other and
be squeezed closer.
Write solution detailed solution (Given, Unknown, Formula, Step-by-Step Solution). Box your final answer. Please make sure that your handwritten is readable. Thank you.
Temperature
1. A copper piece kept at room temperature (20.0°C) has a length of 4.855 um but needs to
reach a length of 4.955 µm for use. What temperature change must be reached to achieve
this new length? Does this change in temperature represent a heating or cooling of the
piece? Show all your work, include correct sig figs and units. Box your final answer.
2. A really big cubic container has an edge length of 8.00 m at a temperature of 72°F. The
container holds air at a pressure of 101 kPa (1.01 x 105 N/m?). How many moles of air fill the
container?
Chapter 13 Solutions
Physics for Scientists and Engineers with Modern Physics
Ch. 13.3 - Prob. 1AECh. 13.3 - A dam holds hack a lake that is 85 m deep at the...Ch. 13.7 - On the hydrometer of Example 1311, will the marks...Ch. 13.7 - Which of the following objects, submerged in...Ch. 13.7 - Which of the following objects, submerged in...Ch. 13.9 - As water in a level pipe passes from a narrow...Ch. 13.10 - Return to Chapter-Opening Question 2, page 339,...Ch. 13 - If one material has a higher density than another,...Ch. 13 - Airplane travelers sometimes note that their...Ch. 13 - The three containers in Fig. 1343 are filled with...
Ch. 13 - Consider what happens when you push both a pin and...Ch. 13 - A small amount of water is boiled in a 1-gallon...Ch. 13 - Prob. 6QCh. 13 - An ice cube floats in a glass of water filled to...Ch. 13 - Will an ice cube float in a glass of alcohol? Why...Ch. 13 - A submerged can of Coke will sink, but a can of...Ch. 13 - Why dont ships made of iron sink?Ch. 13 - Explain how the tube in Fig. 1344, known as a...Ch. 13 - A barge filled high with sand approaches a low...Ch. 13 - Explain why helium weather balloons, which are...Ch. 13 - A row boat floats in a swimming pool, and the...Ch. 13 - Will an empty balloon have precisely the same...Ch. 13 - Why do you float higher in salt water than in...Ch. 13 - If you dangle two pieces of paper vertically, a...Ch. 13 - Why does the stream of water from a faucet...Ch. 13 - Prob. 19QCh. 13 - A tall Styrofoam cup is filled with water. Two...Ch. 13 - Why do airplanes normally lake off into the wind?Ch. 13 - Two ships moving in parallel paths close to one...Ch. 13 - Prob. 23QCh. 13 - Prob. 24QCh. 13 - (I) The approximate volume of the granite monolith...Ch. 13 - (I) What is the approximate mass of air in a...Ch. 13 - (I) If you tried to smuggle gold bricks by filling...Ch. 13 - (I) State your mass and then estimate your volume....Ch. 13 - (II) A bottle has a mass of 35.00g when empty and...Ch. 13 - (II) If 5.0L of antifreeze solution (specific...Ch. 13 - Prob. 7PCh. 13 - (I) Estimate the pressure needed to raise a column...Ch. 13 - (I) Estimate the pressure exerted on a floor by...Ch. 13 - (I) What is the difference in blood pressure...Ch. 13 - (II) How high would the level be in an alcohol...Ch. 13 - (II) In a movie, Tarzan evades his captors by...Ch. 13 - (II) The maximum gauge pressure in a hydraulic...Ch. 13 - (II) The gauge pressure in each of the four tires...Ch. 13 - (II) (a) Determine the total force and the...Ch. 13 - (II) A house at the bottom of a hill is fed by a...Ch. 13 - (II) Water anti then oil (which dont mix) are...Ch. 13 - (II) In working out his principle, Pascal showed...Ch. 13 - (II) What is the normal pressure of the atmosphere...Ch. 13 - (II) A hydraulic press for compacting powdered...Ch. 13 - (II) An open-tube mercury manometer is used to...Ch. 13 - (III) A beaker of liquid accelerates from rest, on...Ch. 13 - (III) Water stands at a height h behind a vertical...Ch. 13 - (III) Estimate the density of the water 5.4 km...Ch. 13 - (III) A cylindrical bucket of liquid (density ) is...Ch. 13 - (I) What fraction of a piece of iron will he...Ch. 13 - (I) A geologist finds that a Moon rock whose mass...Ch. 13 - (II) A crane lifts the 16,000-kg steel hull of a...Ch. 13 - (II) A spherical balloon has a radius of 7.35 m...Ch. 13 - (II) A 74-kg person has an apparent mass of 54 kg...Ch. 13 - (II) What is the likely identity of a metal (see...Ch. 13 - (II) Calculate the true mass (in vacuum) of a...Ch. 13 - Prob. 33PCh. 13 - (II) A scuba diver and her gear displace a volume...Ch. 13 - (II) The specific gravity of ice is 0.917, whereas...Ch. 13 - (II) Archimedes principle can be used not only to...Ch. 13 - (II) (a) Show that the buoyant force FB on a...Ch. 13 - (II) A cube of side length 10.0 cm and made of...Ch. 13 - (II) How many helium-filled balloons would it take...Ch. 13 - Prob. 40PCh. 13 - (III) If an object floats in water, its density...Ch. 13 - (III) A 3.25-kg piece of wood (SG = 0.50) floats...Ch. 13 - (I) A 15-cm-radius air duct is used to replenish...Ch. 13 - Prob. 44PCh. 13 - (I) How fast does water flow from a hole at the...Ch. 13 - (II) A fish tank has dimensions 36 cm wide by 1.0...Ch. 13 - (II) What gauge pressure in the water mains is...Ch. 13 - Prob. 48PCh. 13 - (II) A 180-km/h wind blowing over the flat roof of...Ch. 13 - (II) A 6.0-cm-diameter horizontal pipe gradually...Ch. 13 - (II) Estimate the air pressure inside a category 5...Ch. 13 - (II) What is the lift (in newtons) due to...Ch. 13 - (II) Show that the power needed to drive a fluid...Ch. 13 - (II) Water at a gauge pressure of 3.8 atm at...Ch. 13 - (II) In Fig. 1355, take into account the speed of...Ch. 13 - (II) Suppose the top surface of the vessel in Fig....Ch. 13 - (II) You are watering your lawn with a hose when...Ch. 13 - (III) Suppose the opening in the tank of Fig. 1355...Ch. 13 - Prob. 59PCh. 13 - (III) (a) Show that the flow speed measured by a...Ch. 13 - Prob. 61PCh. 13 - (III) A fire hose exerts a force on the person...Ch. 13 - (II) A viscometer consists of two concentric...Ch. 13 - Prob. 64PCh. 13 - (I) Engine oil (assume SAE 10, Table 133) passes...Ch. 13 - Prob. 66PCh. 13 - (II) What diameter must a 15.5-m-long air duct...Ch. 13 - (II) What must be the pressure difference between...Ch. 13 - (II) Poiseuilles equation does not hold if the...Ch. 13 - Prob. 70PCh. 13 - (III) A patient is to be given a blood...Ch. 13 - (I) If the force F needed to move the wire in Fig....Ch. 13 - (I) Calculate the force needed to move the wire in...Ch. 13 - (II) The surface tension of a liquid can be...Ch. 13 - (III) Estimate the diameter of a steel needle that...Ch. 13 - (III) Show that inside a soap bubble, there must...Ch. 13 - (III) A common effect of surface tension is the...Ch. 13 - A 2.8-N force is applied to the plunger of a...Ch. 13 - Intravenous infusions are often made under...Ch. 13 - A beaker of water rests on an electronic balance...Ch. 13 - Estimate the difference in air pressure between...Ch. 13 - A hydraulic lift is used to jack a 920-kg car 42...Ch. 13 - When you ascend or descend a great deal when...Ch. 13 - Giraffes are a wonder of cardiovascular...Ch. 13 - Suppose a person can reduce the pressure in his...Ch. 13 - Airlines are allowed to maintain a minimum air...Ch. 13 - A simple model (Fig. 13-57) considers a continent...Ch. 13 - A ship, carrying fresh water to a desert island in...Ch. 13 - During ascent, and especially during descent,...Ch. 13 - A raft is made of 12 logs lashed together. Each is...Ch. 13 - Estimate the total mass of the Earths atmosphere,...Ch. 13 - Prob. 92GPCh. 13 - Four lawn sprinkler heads are fed by a...Ch. 13 - A bucket of water is accelerated upward at 1.8 g....Ch. 13 - The stream of water from a faucet decreases in...Ch. 13 - You need to siphon water from a clogged sink. The...Ch. 13 - An airplane has a mass of 1.7 106 kg, and the air...Ch. 13 - A drinking fountain shoots water about 14 cm up in...Ch. 13 - A hurricane-force wind of 200 km/h blows across...Ch. 13 - Blood from an animal is placed in a bottle 1.30 m...Ch. 13 - Prob. 101GPCh. 13 - Prob. 102GPCh. 13 - A two-component model used to determine percent...Ch. 13 - (III) Air pressure decreases with altitude. The...
Additional Science Textbook Solutions
Find more solutions based on key concepts
While camping, you boil water to make spaghetti. Your pot contains 2.5 kg of water initially at 10C. You stoke ...
Essential University Physics (3rd Edition)
Express the unit vectors in terms of (that is, derive Eq. 1.64). Check your answers several ways Also work o...
Introduction to Electrodynamics
How did the Copernican revolution alter perceptions of the ancient Greek debate over extraterrestrial life? (a)...
Life in the Universe (4th Edition)
Calculate the mass of a mole of dry air, which is a mixture of N2(78%byvolume),O2(21%), and argon(1%).
An Introduction to Thermal Physics
1. When is energy most evident?
Conceptual Physics (12th Edition)
The pV-diagram of the Carnot cycle.
Sears And Zemansky's University Physics With Modern Physics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The cylinder in (Figure 1) is divided into two compartments by a frictionless piston that can slide back and forth. Assume the piston is in equilibrium. Figure 80°C Piston 20°C 1 of 1 > Part A Is the pressure on the left side greater than, less than, or equal to the pressure on the right? Explain. Match the words in the left column to the appropriate blanks in the sentences on the right. greater than less than the pressure is higher at the higher temperature Submit equal to the separated gases tend to occupy the same volume Provide Feedback equal forces act on the piston from the left and from the right Request Answer The pressure on the left side is the pressure on the right side because Reset Helparrow_forwardShow your complete solution and illustrate if applicable. box the final answer. Please write your solution clearly and readable. Thank you.arrow_forwardPlease answer all included questions. PLEASE! Do not skip any steps. Include all work with notes associated with the correct formulas. Please double check your work. Thank you!arrow_forward
- In first step please write main concept or expression.please explain brieflyarrow_forwardThe mechanism shown in (Figure 1) is used in a riveting machine. It consists of a driving piston A, three links, and a riveter which is attached to the slider block D Part A Determine the x and y components of the velocity of D at the instant shown, when the piston at A is traveling at vA = 12 m/s Express your answers using three significant figures separated by a comma. VO AEo n vec (vD)z, (vD)y = m/s Submit Request Answer Figure > 45° 300 mm 150 mm 45 200 mm 30 60 45°arrow_forwardProvide a brief solution in problem in the picture below.In picture below, there's an answer key for the problem. You must answer a problems in letter a and b in picture. Your answer in letter a must be the same in an answer key provided below. Thank you in advance.arrow_forward
- Would like answers for part B and C ONLY please-need info from part a to answer them. I already solved part a) 1)Energy Efficiency at Home : Suppose you have two kettles – a plug-in electrickettle and a stovetop kettle. The electric kettle uses electricity from a natural gas fired powerplant, while you boil water in your stovetop kettle on a natural gas burner. a)Based on the following information, which of these kettles demonstrates a higher energy efficiency (i.e., which is more efficient at using energy from fuel to heat water)? Please calculate the efficiencies of each kettle and express answers as percentages. -The natural gas power plant converts chemical energy of the natural gas to electrical energy with 58% efficiency. -High-voltage power lines from the power plant to your house convey electricity with 92% efficiency. -The electric kettle converts electrical energy to thermal energy in the water with 85% efficiency (the other 15% heats up the kettle itself). -The stove…arrow_forwardSen d na IQuestion & of4 Question 4 Note: Round off your answer to the nearest whole number. Input the numerical value only. Problem. A composite wire to be used as an integral component of a switch in an electrical device is composed of wire A and wire B. The wire has effective linear expansion coefficient of 0.00002 per Celslus-degree. What percentage of the total iength of the composite wire is wire B? The linear expansion coefficient of wire A is 0.000016 per Celsius-degree. The linear expansion coefficient of wire B is 0.000036 per Celsius-degree. MacBook Proarrow_forwardTRUE OR FALSE. On the answer sheet, shade the circle for Letter A if the statement is correct. Otherwise, shade the circle for Letter E. I. 1. Boat floats because there is a force that is pushing it up. 2. Only the weight of the object determines if the object will sink or float. 3. If I change the shape of the clay, its weight and volume also change. 4. Cutting a wood in half will also make its density half of the original density. 5. The pressure exerted by the fluid at rest on an object is directly proportional to the density of the liquid and depth and is sometimes called as gauge pressure.arrow_forward
- A person consumes about 2200 Cal a day. 1 Cal = 4186 J, and 1 kWh = 860 Cal. Part A What is this energy in joules? Express your answer using two significant figures. for Part A for Part A do for Part redo for Part A reset for Part A keyboard shortcuts for Part A help for Pal 2=9.6.106 Submit Previous Answers Request Answer X Incorrect; Try Again; One attempt remaining Review your calculations and make sure you round to 2 significant figures in the last step.arrow_forwardI need more detail about my answer, so watch the professor's comment and write your solution more detail. 2. A cylindrical object—with radius R, length L, and density ??—sits on a scale at the bottom of vat of fluid with density??.a. Draw and label a free body diagram for the object. (Don’t forget to set up a coordinate system and to label the acceleration info. Also, be sure to use the gridlines to help you draw arrows that show the correct directions and relative magnitudes of the forces. Finally, be sure to label your forces clearly to indicate the types of forces.)b. Use Newton’s 2nd Law to derive an expression for the reading on the scale.arrow_forwardA balloon, in the shape of a right circular cylinder, is being inflated in such a way that the radius and height are both increasing at the rate of 3 cm/s and 8 cm/s, respectively. What is the rate of change of its total surface area when its radius and height are 60 cm and 140 cm, respectively?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY