
(a)
Interpretation:
The electronic partition function of
Concept introduction:
Statistical
(a)
Answer to Problem 13C.7P
The electronic partition function of
| Temperature | Partition |
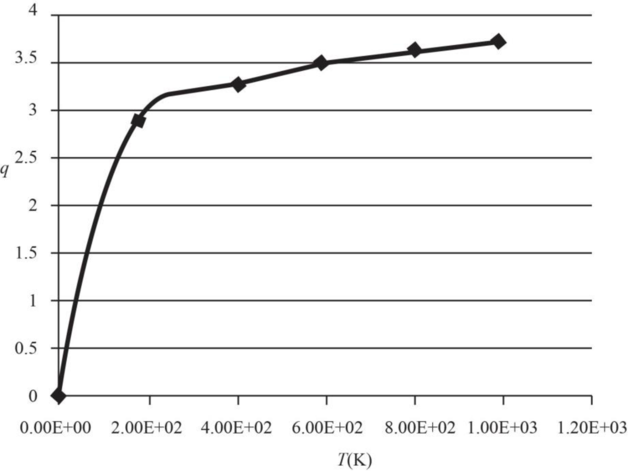
The graph of electronic partition function of
The population of ground and first excited levels of
Explanation of Solution
The partition function in terms of wavenumber is given by the expression as shown below.
Where,
The degeneracy of the ground and first excited electronic level of
The wavenumber of first excited electronic level of
The wavenumber of ground electronic level of
The temperature range is from
The partition function of
Substitute the values of
Substitute the value
Similarly, the partition is calculated for the temperature range from
| Temperature | Partition |
Therefore, the plot electronic partition function of
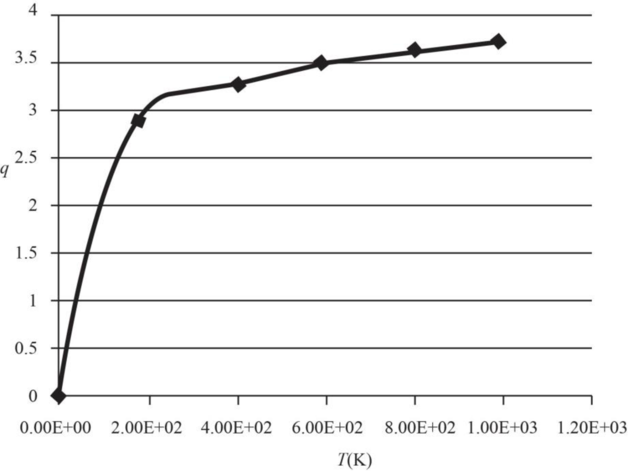
Figure 1
The relative population of ground level of
Substitute the values of
When the total population of the system is
The pollution of first excited level of
Where,
Substitute the value of
Therefore, the population of ground and first excited levels of
(b)
Interpretation:
The mean electronic energy of
Concept introduction:
Refer to part (a).
(b)
Answer to Problem 13C.7P
The mean electronic energy of
Explanation of Solution
The degeneracy of the ground and first excited electronic level of
The wavenumber of first excited electronic level of
The wavenumber of ground electronic level of
The temperature is
The partition function of
Where,
The mean energy,
Substitute the value of
Substitute the value of
Where,
Substitute the values of
The above expression can be further simplified as shown below.
Therefore, the mean electronic energy of
Want to see more full solutions like this?
Chapter 13 Solutions
Atkins' Physical Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





