
Concept explainers
(a)
Interpretation:
The molar mass of the compound is to be calculated.
Concept introduction:
The freezing point is the temperature at which both the solid and liquid phases coexist in equilibrium. It is the temperature at which the vapor pressure of the substance in the liquid state becomes equal to the vapor pressure in a solid state.
The formula to calculate the change in freezing point is as follows:
Here,
(a)
Answer to Problem 13.128P
Explanation of Solution
The formula to calculate the change in freezing point is as follows:
Substitute
The solute is a nonvolatile non-electrolyte so its van’t Hoff factor is 1.
Rearrange equation (1) to calculate the molarity of the solution as follows:
Substitute 1 for
The density of the solution is calculated as follows:
Rearrange equation (4) to calculate the mass of the solution as follows:
Substitute
The formula to calculate the molality of the solution is as follows:
Rearrange equation (6) to calculate the moles of solute as follows:
Substitute
The formula to calculate the number of moles is as follows:
Rearrange equation (8) to calculate the molar mass as follows:
Substitute
(b)
Interpretation:
The empirical and the molecular formula of the compound are to be determined.
Concept introduction:
An empirical formula gives the simplest whole number ratio of atoms of each element present in a molecule. The molecular formula tells the exact number of atoms of each element present in a molecule.
(b)
Answer to Problem 13.128P
The empirical and molecular formula of the compound is
Explanation of Solution
Consider the mass of the compound to be
The formula to calculate the mass of the compound is as follows:
Rearrange equation (10) to calculate the mass of oxygen as follows:
Substitute
The formula to calculate the moles of the compound is as follows:
Substitute
Substitute
Substitute
Write the amount of carbon, hydrogen, and oxygen as subscripts of their symbols to obtain a preliminary formula as follows:
The smallest subscript is
The subscripts are in the whole number. Hence, the empirical formula of the compound is
The expression to calculate the empirical formula mass of
Substitute
The molar mass of the compound is
(c)
Interpretation:
The Lewis structures for the compound that forms hydrogen bonds and one that does not form hydrogen bonds are to be drawn.
Concept introduction:
Lewis structure is basically a simplified representation of the structure of any molecule or atom. Lewis structure shows the bonding between the atoms and the lone pairs of electrons as dot.
The steps to draw the Lewis structure of any molecule are as follows:
1. Write the letter
2. Count the total number of valence electrons in the molecule. In case of charged molecules subtract the positive charge from the total number of valence electrons and add the negative charge to the total number of valence electrons.
3. Assign two electrons between two atoms and join them via a single bond. Place the remaining valence electrons as lone pairs such that octet of each element is achieved. Use multiple bonds to complete the octet.
(c)
Answer to Problem 13.128P
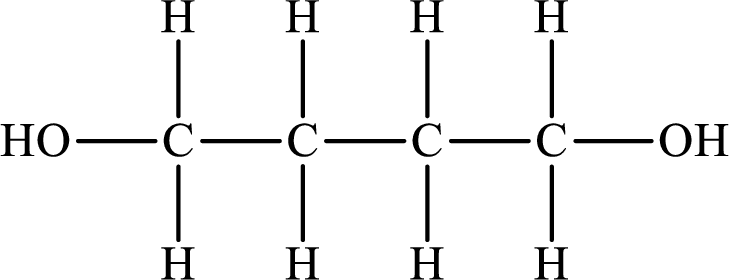
The Lewis structure of the compound that forms hydrogen bonds is as follows:
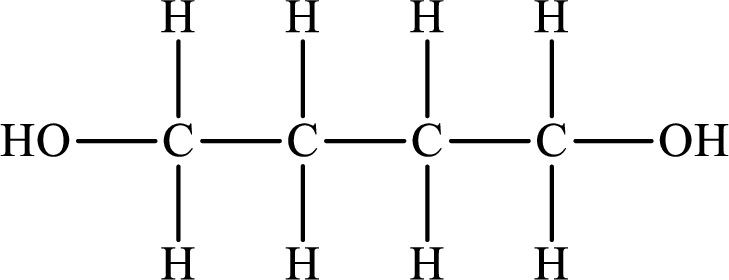
The Lewis structure of the compound that does not form hydrogen bonds is as follows:
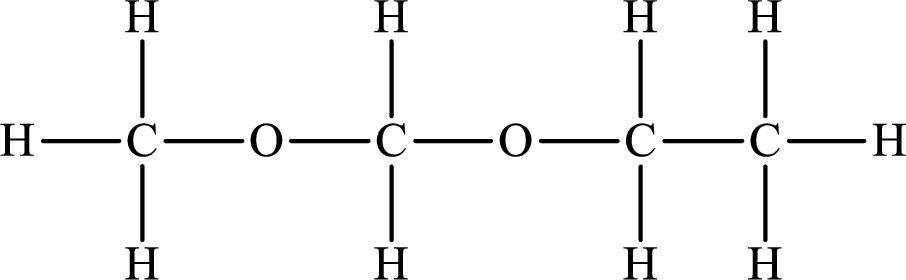
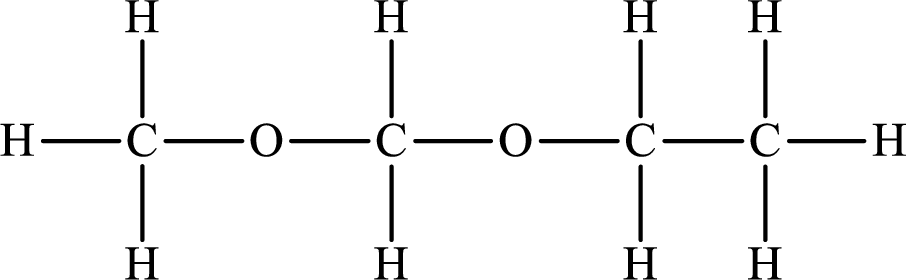
Explanation of Solution
The compound will form hydrogen bonds with the electronegative element is present at the end positions. But no hydrogen bonding occurs when the electronegative element is placed in between the other elements.
So the Lewis structure of the compound that forms hydrogen bonds is as follows:
The Lewis structure of the compound that does not form hydrogen bonds is as follows:
Want to see more full solutions like this?
Chapter 13 Solutions
MCGRAW: CHEMISTRY THE MOLECULAR NATURE
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





