
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.5, Problem 12.2P
Which of the bonds shown in red are expected to have IR-active stretching frequencies?
- (a) H—C≡C—H
- (b) H—C≡C—H
- (c) H—C≡C—CH3
- (d) H3C—C≡C—CH3
- (e) H3C—C≡C—CH3
- (f) H3C—CH3
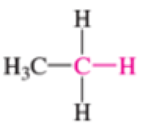
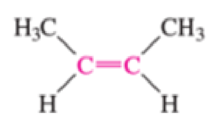
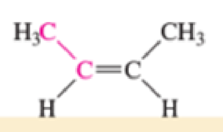
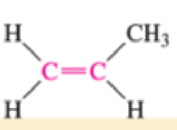
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. What are the spin system of the following compounds:
(a)
Cl₂CHCH2CHC₁₂
(b) CF3C=C-H
(c)
CI-
H
H
H
H
-Br
(d)
H3C
H
CH3
Which would you expect to have higher frequency C=O stretching vibration?
H
ہو
H
H
H
—F
F
or each of the following molecules, construct the MOS
from the 2p, atomic orbitals perpendicular to the plane of
the carbon atoms.
(a) Cyclobutadiene HC=CH
НС—СН
HC=CH
НС —СH
(b) Allyl radical
H
H
H
H
C=C
•C-C,
H
C-H
H
C-H
H
H
Indicate which, if any, of these orbitals have identical ener-
gies from symmetry considerations. Show the number of
electrons occupying each w MO in the ground state, and
indicate whether either or both of the molecules are para-
magnetic. Assume that the C atoms in the allyl radical are
all sp? hybridized.
Activate
io to S
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Assign the configuration Z or E to each double bond, where appropriate, in the following molecules. (a) (b) (c) Br Br NO2arrow_forwardA chair structure of a trisubstituted cyclohexane is shown below. Determine which of the following 2D representations matches the chair structure. CH3 CH3 H3C- CH3 A) I II CH3 CH3 CH3 B) I| CH C) II CH3 IV CH, CH3 CH3 D) IVarrow_forwardSelect a single sp3–sp3 or sp3–sp2 bond from a linear portion of the molecule, identify it, and draw a Newman projection for it. Then draw a Newman projection for a 60 degree rotational isomer. List each rotational isomer as lower or higher energy. If your molecule is cyclic and has no linear portions, then draw the expected conformation of the cyclic portion of the molecule.arrow_forward
- 7. Assign E or Z configuration to the following molecules: (A) (B) (C) (D) (E) I = Z; II = E I=E: II = Z I= E; II = E I=Z; II = Z I= E; II is neither E nor Z OH I II C1arrow_forward(a) How many π molecular orbitals are present in deca-1,3,5,7,9-pentaene (CH2 = CH – OH = OH – CH = CH – OH = OH – CH = CH2)? (b) How many are bonding MOs and how many are antibonding MOs? (c) How many nodes are present in ψ1? (d) How many nodes are present in ψ10*arrow_forwardThe degree of unsaturation, or index of hydrogen deficiency, is the number of pi bonds plus rings in a molecule. Specify the degree of unsaturation (index of hydrogen deficiency) of the following formulas: (a) C9H12 (b) C,H40| (c) C,H,N2arrow_forward
- 8. Which of the following pairs of compounds is likely to absorb radiation at the longer wavelength and with greater intensity? (a) CH3CH2CO₂H or CH2=CHCO₂H (b) CH3CH=CHCH=CHCH3 or CH3C = C—C = CCH3 OCH3 (c) or CH3arrow_forward2. Provide the approximate wavenumber for the IR absorption band from stretching vibration of bond identified in each structure. || -CEN H TIL H javarrow_forward2. Which of the following compounds would you expect to have the highest infrared absorption frequency C-X bond? Bubble in your answer completely. O Br O O Oarrow_forward
- Choose the factor that explains this observation. || A) resonance stabilization B) charge C) electronegativity D) polarizability OH OH₂arrow_forwardwhich of the following compounds absorb the radiation at longer wave length, Why ? 1- H;C-CH CH-CH=CH-CH3 2- H,C CH CH-CH=CH-C-CH3arrow_forwardHow do the following factors affect absorption frequencies? O-H vs O-D. CH stretch, hybrid orbitals used by carbonarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY