
Interpretation:
For each transformation, the reducing agent used, out of
Concept introduction:
Lithium aluminium hydride and sodium borohydride are reducing agents.
Answer to Problem 1PP
Solution:
(a)
(b)
(c)
(d)
(e)
(a)
Explanation of Solution
The given transformation is as follows:
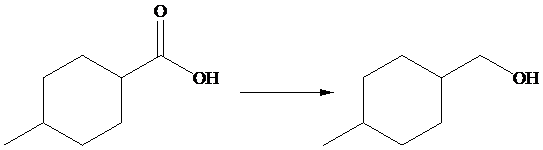
In the above transformation,
Hence,
(b)
The given transformation is as follows;
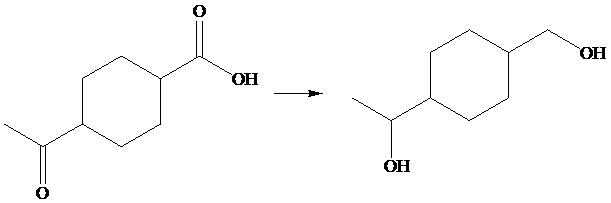
In the above transformation, carboxylic acid and ketone are converted into their corresponding alcohols. This transformation is done by using
Hence,
(c)
The given transformation is as follows:
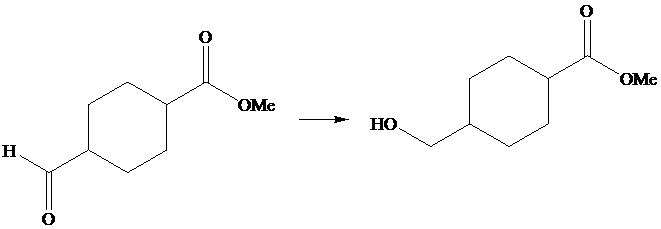
In the above transformation, aldehyde is converted into its corresponding alcohol, but there is no effect on the ester group. This transformation is done by using
Hence,
(d)
The given transformation is as follows:
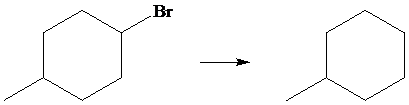
In the above transformation, the bromine atom is replaced by the hydrogen atom. This transformation is done by using
Hence,
(e)
The given transformation is as follows:
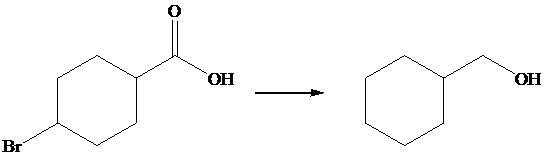
In the above transformation, the bromine atom is replaced by the hydrogen atom, and carboxylic acid is converted into its corresponding alcohol. This transformation is done by using
Hence,
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
- 4. (A) A medicinal chemist wished to make a series of aromatic molecules bearing a ketone and thioether group with ortho, meta, or para relationships. To achieve this, they propose using nucleophilic aromatic substitution treating the corresponding ortho, meta, and para aryl chlorides with sodium ethanethiolate (NaSEt). Predict which of these reactions will likely work and which will likely fail. Provide a mechanistic explanation why. SET O NasEt EtS glagol Glal ol heat EtS target molecules EtS (B) Would the analogous reactions using EtMgBr instead of NaSEt be more or less likely to work? Explain why or why not. لمسلم EtMgBrarrow_forwardUse retrosynthetic notation to show the combination of alkyl bromide and potassium alkoxide that would be the most effective in the syntheses of the following ethers. (a) CH3OC(CH3)3(b) (CH3)3CCH2OCH2CH3arrow_forward(b) Give reagents and conditions that will allow ALL of the following synthetic transformations to be achieved. More than one synthetic step may be required in each case. You may assume that any required additional reagents will be available. HO OH e (1) (ii) 6 (iii) -6 O-SiMe3 O₂N- O₂N OH (c) Give reagents and conditions that will allow the following synthetic transformation to be achieved using organosilicon chemistry. More than one synthetic step will be required. Ph-H CH₂CH3 Ph Aarrow_forward
- (a) Give the names of reagents and conditions for each of the reactions I and IIarrow_forward(b) Outline, using suitable mechanisms, how the following conversion may be brought about. You should indicate the reagents to be used and any intermediate compounds that may be formed. Each arrow may represent one or more than one step. P(C6H5)3 HC 8-6-30 CH₂Br HC H CH3arrow_forward13. Starting with benzene provide a multi-step synthesis of the molecules shown below. You may use any needed inorganic reagents. (a) (b) OH HO 0-10- Brarrow_forward
- Provide reasonable step-by-step mechanisms for the following reactions. Use arrows to indicate themovement of electrons.arrow_forwardPropose structural formulas for compounds A, B, and C in the following conversion. Also show how to prepare compound C by a Wittig reaction. 1. HC=CH, NANH, KHSO4, C-H10 C,H1,0 C,H120 2. Н,О Lindlar heat catalyst (A) (B) (C)arrow_forwardCompounds containing deuterium (D = 2H) are useful for kinetic studies and metabolic studies with new pharmaceuticals.One way to introduce deuterium is by using the reagent LiAlD4, equivalent in reactivity to LiAlH4. Show how to makethese deuterium-labeled compounds, using LiAlD4 and D2O as your sources of deuterium, and any non-deuterated startingmaterials you wish.(a) CH3CHDOH (b) CH3CD2OH (c) CH3CD2ODarrow_forward
- 3. Outline a synthetic scheme for the preparation of the compounds A from the suggested starting material by the suggested method. Make sure to clearly indicate all reagents needed to perform each step of the synthetic scheme. (a) OH O=S=O Br A (b) H₂N. (c) HO3S. Br Br A A 1 Brarrow_forwardWhich of the following compounds will undergo an SN2 reaction more easily? (a) neopentyl iodide (b) tert-butyl chloride (c) 2-iodopropane (d) 4-methyl-1-iodopentane (e) 1-chloro-4-methylpentane. Briefly justify your answer. Which of the mentioned compounds will undergo an SN1 reaction more easily? Briefly justify your answer.arrow_forwardb) Suggest the appropriate reducing agents for the following transformation. Provide the reason for your choice of reagents. ОН OH -CH3 -CH3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





