
Interpretation: The reagents used to accomplish the given transformations should be identified.
Concept Introduction:
Addition Reaction: It is defined as
Elimination Reaction: It is just reverse reaction of addition where substituent from the given molecule is removed via E1 (the reaction depends only on the substrate involved in the reaction) or E2 (the reaction depends on both of the substituents in the reaction) mechanism.
Substitution Reaction: The reaction in which one group gets substituted by other group. The types of substitution reactions are electrophilic and nucleophilic substitution reactions.
Anti-Markovnikov’s Addition Rule: The unsymmetrical
To identify: The reagents used to complete the given transformations.
Answer to Problem 17PP
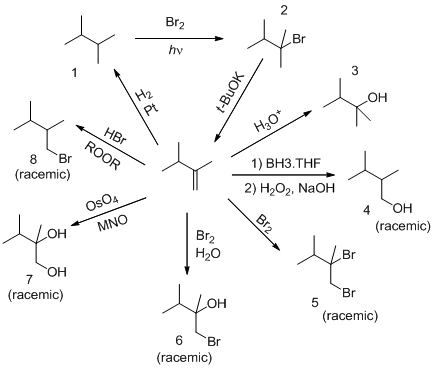
Explanation of Solution
Identify the reagents used in formation of products 1,2 and 3 from given substrate.
Analyzing the given product (1) shows that it lacks carbon-carbon double bond with respect to its reactant. Therefore, the given substrate undergoes reduction in presence of reagents
The given product 2 contains one extra bromine atom when comparing with 1 since product 1 undergoes halogenation reaction. Hence the suitable reagent used in this transformation is
In presence of tertiary butoxide the bromine group in product 2, gets eliminated and the tertiary butoxide abstracts protons from one carbon which finally results in the formation of given substrate.
Then examining product 3 shows that the carbon in carbon-carbon double bond in given substrate is protonated to give more stable carbocation and finally the nucleophile
Identify the reagents used in formation of products 4,5 and 6 from given substrate.
Examining product 4 shows that the substrate should undergoes anti-markovnikov addition of
Analyzing the product 5 clearly shows that it contains two bromine atoms extra which is substituted in the carbon-carbon double bond in given substrate which shows that suitable reagent for the transformation is
The product 6 shows that it contains one bromine atom and one
Identify the reagents used in formation of products of 7 and 8 from the given substrate.
Going through the product shows that it contains two hydroxyl groups hence suitable reagent for this transformation is
Product 8 shows that it contains bromine atom in less substituted atom which shows that anti-markovnikov addition happens hence suitable reagent for that is
The reagents used in the given transformations are identified by using the reaction in which the given substrate undergoes
Want to see more full solutions like this?
Chapter 12 Solutions
ORGANIC CHEMISTRY GGC>CUSTOM<-TEXT
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





