
Concept explainers
Interpretation:
The following compounds are to be matched with their respective IR spectra.
(a)
(b)
(c)
(d) dipropyl ether
(e)
(f) cyclohexane
(g)
Concept introduction:
The IR spectrum of a compound shows absorptions at different frequencies. These absorption frequencies depend upon the bond stretching frequency of the bonds in molecule.
Answer to Problem 12.27AP
The matching of the given compounds with their respective IR spectra is shown in the table below.
| Compounds | IR spectra |
| a. |
 |
| b. |
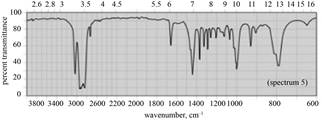 |
| c. |
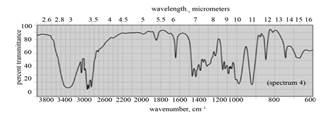 |
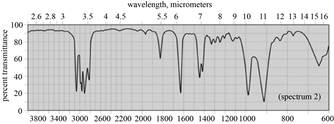
| d. dipropyl ether | 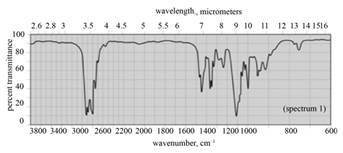 |
| e. |
No IR spectrum available. |
| f. cyclohexane | 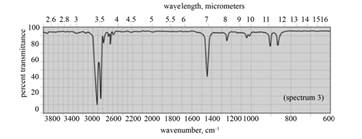 |
| g. |
No IR spectrum available. |
Explanation of Solution
(a) The given compound is
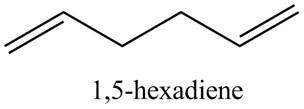
Figure 1
In the IR spectra of
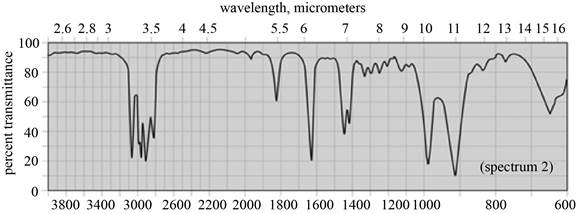
Figure 2
(b) The given compound is
Figure 3
In the IR spectra of
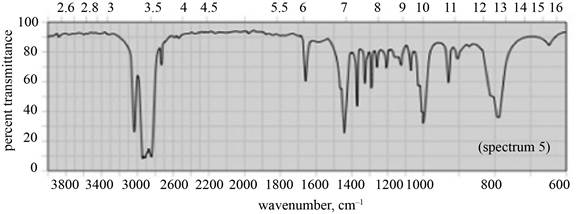
Figure 4
(c) The given compound is
Figure 5
In the IR spectra of
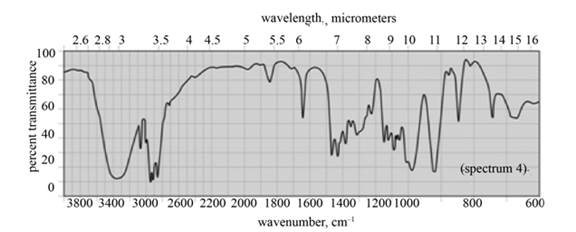
Figure 6
(d) The given compound is dipropyl ether,
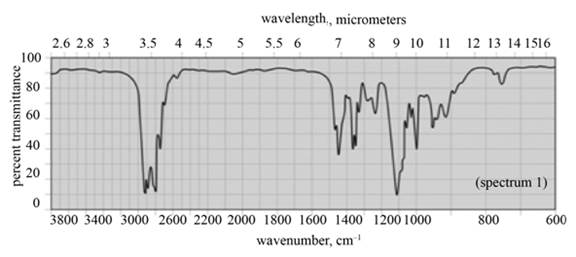
Figure 7
(e) The IR spectrum for
(f) The given compound is cyclohexane as shown below.
Figure 8
In the IR spectra of cyclohexane, a strong absorption peak for
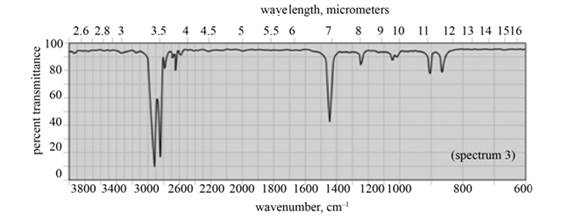
Figure 9
(g) The IR spectrum for
(a) The spectrum 2 corresponds to the compound,
(b) The spectrum 5 corresponds to the compound,
(c) The spectrum 4 corresponds to the compound,
(d) The spectrum 1 corresponds to the compound, dipropyl ether.
(e) There is no spectrum available for the compound,
(f) The spectrum 3 corresponds to the compound, cyclohexane.
(g) There is no spectrum available for the compound,
Want to see more full solutions like this?
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY
- 2. (18pts) (a) Draw bond-line structures and include appropriate wedge and dash notation for the following compounds. (b) Identify each pair that would be optically inactive as a 50/50 mixture. (c) Identify pair of compounds that represent diastereomers. H H A Br Br Br H B Br Br H-Et H Br C Br Br CH3 D H Harrow_forwardНас 3. (5S,7R) -7-bromo-3,9-diethyl-6,6-dimethylundec-3,8-dien-5-ol H C Н.С Ay statuvos OH CH3 Ay si Br Н CH3 CH3 I b. number of peaks = С.arrow_forward(a) The 'H-NMR spectrum of cyclobutanone shows two signals - signal A at 3.00 ppm and signal B at 1.95 ppm. Give the multiplicity of each signal. cyclobutanone (b) When cyclobutanone is treated with D20 and NaOD, the only signal observable in the 1H-NMR is a singlet at 2.00 ppm. Explain why this is the case. [Note: Deuterium atoms do not display signals in the TH-NMR spectrum]arrow_forward
- 1. Deduce a possible structure for the compound with the IR absorptions below. (a) C5H8O: 2950, 1750 cm-1 (b) C4H8O: 2950, 2820, 2715, 1715 cm-1 2. How could IR spectroscopy be used to distinguish between the following pair of compounds? (a) CH2=CHCH2CH(CH3)2 and CH3CH2CH2CH(CH3)2 (b) CH3OCH2CH3 and CH3CH2CH2OH 4. How does the O-H stretch in the IR spectrum of a carboxylic acid differ from the O-H stretch of an alcohol?arrow_forwarda) b) Can these three molecules shown below be distinguished by ¹H NMR spectroscopy when the NMR spectrum is recorded in anhydrous (water-free) CDCI3? Give reasons for your answer. NH₂ Can these three molecules shown below be distinguished by ¹H NMR spectroscopy when the NMR spectrum is recorded in deuterated water (D₂O)? Give reasons for your answer. NH₂arrow_forward(a) A compound known to be a substituted cyclohexanone derivative has lamda max of 235 nm. Could this compound be a conjugated dienone? explain (b) (i)For this compound, how many nm must be accounted for by substituents? (ii) What are the substituents and the points of substitution that may occur having accounted for the 20nm?arrow_forward
- 2 (a) In the following reaction, PCC A (i) Draw the structure of compound A. (ii) Explain using the IR spectra to confirm that the reaction is completed. (iii) Identify the molecular ion peak for compound A. (iv) Fragmentation of A shows a peak at m/z 111. Draw the possible cation for this peak. (v) Determine the resonance structure of this cation.arrow_forwardFor each of the molecules below: (a) Provide the bond line structures (b) Indicate the number of peaks that would be seen in their ¹H-NMR spectra. Number each unique type of hydrogen peaks as shown in the example on the right. (c) Label the diasterotopic and enantiotopic atoms and/or groups. 1. (1R,4S) 4-secbutyl-2,3-diethyl-6,6-dimethylcyclohex-2-enol 2. (2R,6S) 2,6-dibromo-4,4-dimethylcyclohexanol 3. (5S,7R) -7-bromo-3,9-diethyl-6,6-dimethylundec-3,8-dien-5-ol Example: 1 2 1Η 2arrow_forward(a) What would be the frequencies of the two absorption bands expected to be most prominent in the infrared spectrum of 4-hydroxycycloheptanone (C)? (b) In reality, the lower frequency band of these two is very weak. Draw the structure of an isomer that would exist in equilibrium with C and that explains this observation.arrow_forward
- you infrared spectroscopy. (a) (b) H distinguish between the following pairs of compounds by using NH2arrow_forward(a) Which molecule shown below is most consistent with the H-NMR spectrum that is shown? (b) Label the C-H in the molecule of your choice, then match the peaks in the NMR spectrum with proper label of the C-H. (Do it on a piece of paper, then scan it into PDF file and upload.) (a) (b) (c) (d) TMS ille 4.400 4.200 4.000 3.800 3.600 3.400 3.200 3.000 2.800 2.600 2.400 2.200 2.000 1.800 1.600 1.400 1.200 1.000 0.800 0.600 0.400 0.200 0.00arrow_forwardCompound E with a molecular formula C8H7CIO shows a prominent band in its IR spectrum at 1690 cm-1. 'H NMR spectrum revealed only two major types of protons in the ratio of 5: 2. 2 (i) Calculate the degree of unsaturation for compound E. (ii) By using the IR and 'H NMR data given, deduce the structure of compound E.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





