
Interpretation:
Ionic atmosphere has to be defined.
Concept Introduction:
Ionic solutions are characterized by presence of numerous cations and anions. Each cation and anion interacts with one another via electrostatic attractive forces. Such ionic spheres composed of cation surrounded by anions or vice versa are present throughout the medium of ionic solutions.
Explanation of Solution
Anion is surrounded by more number of cation than anions, similarly cation are surrounded by more anions than cation. This generates a sphere of net charge around each cation or anion.
Ionic atmosphere may refer to certain positively charged region around anion or negative charged region around a cation. It develops due to mutual interaction through electrostatic forces illustrated as follows:
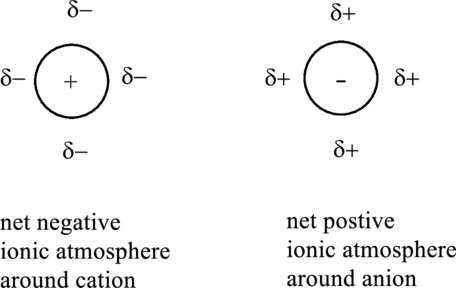
A pure cation or anion is not able to attract each other effectively due to the hindrance caused by ionic atmosphere around them.
Degree of charge concentrated within an ionic atmosphere is governed by extent of concentration of ions. There is continuous diffusion of various ions across the ionic atmosphere.
Want to see more full solutions like this?
Chapter 12 Solutions
Exploring Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





