
Concept explainers
For each compound. [1] Identify the
![Chapter 11.5, Problem 11.8PP, For each compound. [1] Identify the functional group; [2] draw out the complete compound, including , example 1](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9781259883989/Chapter-11/images/html_83989-11.5-11.8pp_image001.jpg)
b.
![Chapter 11.5, Problem 11.8PP, For each compound. [1] Identify the functional group; [2] draw out the complete compound, including , example 2](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9781259883989/Chapter-11/images/html_83989-11.5-11.8pp_image002.jpg)
d.
![Chapter 11.5, Problem 11.8PP, For each compound. [1] Identify the functional group; [2] draw out the complete compound, including , example 3](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9781259883989/Chapter-11/images/html_83989-11.5-11.8pp_image003.jpg)
(a)
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
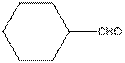
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 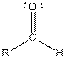 |
| Ketone | 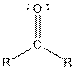 |
| Carboxylic acid | 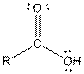 |
| Ester | 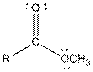 |
| Amide | 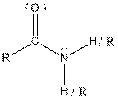 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound is '
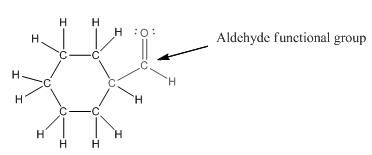
Explanation of Solution
The given compound is as follows:
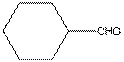
The given compound can also be drawn as:
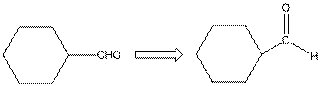
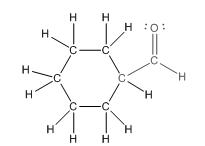
This compound contains a six membered ring and a
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four). Valence electron of oxygen is six. Out of six two electrons are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair.
So, complete structure of given compound is as follows:
(b)
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 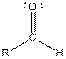 |
| Ketone | 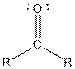 |
| Carboxylic acid | 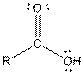 |
| Ester | 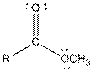 |
| Amide | 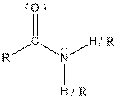 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound
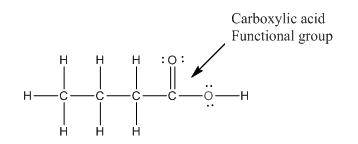
Explanation of Solution
The given compound is as follows:
The above compound can be drawn as:
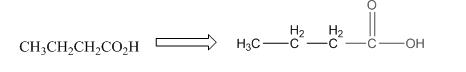
This compound contains four carbon and eight hydrogen atom with two oxygen atoms. The functional group is carboxylic acid containing an
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four). Valence electron of oxygen is six. Out of six two electrons of blue colored oxygen are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair.
Complete structure of the given compound is as follows:

(c)
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 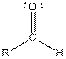 |
| Ketone | 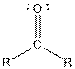 |
| Carboxylic acid | 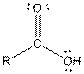 |
| Ester | 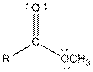 |
| Amide | 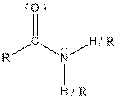 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound is 'ketone' and the complete structure of the compound is as follows:
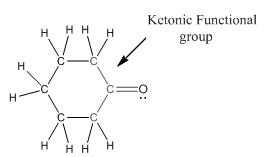
Explanation of Solution
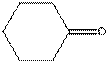
The given compound is as follows:
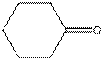
This compound contains six membered ring with one oxygen atom. The functional group is ketone containing a carbon-oxygen bond
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four). Valence electron of oxygen is six. Out of six two electrons of are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair.
Complete structure of the compound is as follows:
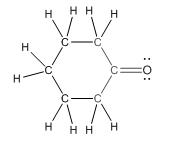
(d)
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 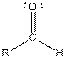 |
| Ketone | 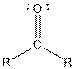 |
| Carboxylic acid | 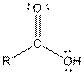 |
| Ester | 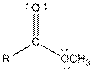 |
| Amide | 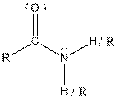 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound is 'ester' and the complete structure of the compound is as follows:
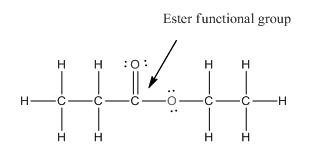
Explanation of Solution
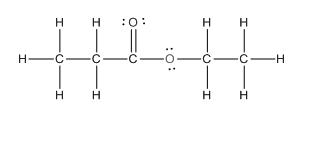
The given compound is as follows:
This given compound can be drawn as follows:
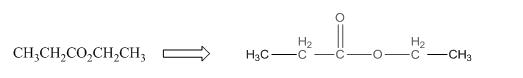
This compound contains an
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four).
Valence electron of oxygen is six. Out of six two electrons of blue colored oxygen are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair. For pink colored oxygen atom, one electron is used in making bond with carbonyl carbon atom and another electron is used in making bond with carbon backbone. Then remaining four will be present as lone pair.
The complete structure of the given compound is as follows:
(e)
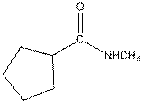
Interpretation:
The functional group / groups should be identified and the complete structure with lone pairs on hetero-atoms of the following compound should be drawn:
Concept Introduction:
Organic molecules have some structural features in addition to
This table gives information about the compounds containing
| Type of Compound | General Structure |
| Aldehyde | 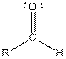 |
| Ketone | 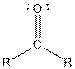 |
| Carboxylic acid | 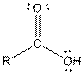 |
| Ester | 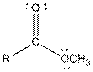 |
| Amide | 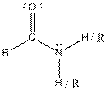 |
Here, R = any carbon backbone
Complete structural formula of a molecule represents all the atoms of molecule, types of bonds connecting atoms and how atoms are connected to each other.
Answer to Problem 11.8PP
Functional group in the given compound is an 'amide' and the complete structure of the compound is as follows:
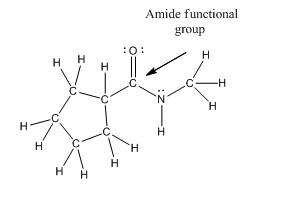
Explanation of Solution
The given compound is as follows:
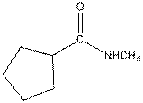
This compound contains an
To draw the complete structure of the given skeletal structure, place carbon atom on each vertices and fulfill its valency with hydrogen atoms. Unlabelled vertex will represent carbon atom attached to number of hydrogen atoms required to fulfill its valency (i.e. four).
Valence electron of oxygen is six. Out of six two electrons of oxygen atom are used in making double bond with carbon atom. Then remaining four electrons will be available as lone pair. Valence electron of nitrogen is five. Out of five, one electron is used in making bond with the carbonyl carbon atom, one electron is used in making bond with
The complete structure of the given compound is as follows:
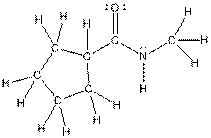
Want to see more full solutions like this?
Chapter 11 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- Which of the following compounds is a valid Lewis structure of a hydrocarbon? H H-C-Ö-H H HH H-C-N-H H I II H H-C-C-H H III HH H-C-C-H I НН IV I, II, and IV are valid structures of hydrocarbons. Only III is a valid structure of hydrocarbons. All of these compounds are valid structures of hydrocarbons. Only IV is a valid structure of hydrocarbons. III and IV are valid structures of hydrocarbons.arrow_forwardConvert each condensed formula to a Lewis structure.1.) (CH3)2CHOCH2CH2CH2OH2.) CH3(CH2)2CO2C(CH3)3arrow_forwardConvert each shorthand structure to a complete structure with all atoms and lone pairs drawn in. a. (CH3)2CH(CH,),CH3 b. (CH3)3COH c. CH;CO2(CH2)3CH3 ÇI CI d. HO OCH(CH3)2arrow_forward
- Convert each molecule into a skeletal structure. a. (CH3)2CHCH,CH;CH(CH3)2 c. CH3(CH2),C(CH3)½CH(CH3)CH(CH3)CH(Br)CH3 нн HT TH C-C H CH3 d. CH3-C C-C C-C b. CH3CH(CI)CH(OH)CH3 CH2 H limonene (oil of lemon)arrow_forwardConvert each molecule into a skeletal structure. a. (CH3)½CHCH,CH2CH(CH3)2 c. CH3(CH2)½C(CH3)½CH(CH3)CH(CH3)CH(Br)CH3 нн HT TH ċ-C H CH3 c-c CH2 b. CH;CH(CI)CH(OH)CH3 d. CH3-C H limonene (oil of lemon)arrow_forwardB. Cycloalkanes 1) Construct a model of the cyclic alkane: cyclopentane (C5H₁0). Because the five carbon atoms are locked in a ring, rotation about the single bonds is restricted; the plane of the ring a fixed geometry within the molecule. Toggle between full Lewis structures and skeletal structures by clicking the C-H tool. Model 1: Use the solid wedge tool to attach a methyl group to each of two different carbon atoms in the cyclopentane. Note the five carbons of the ring are in the plane of the paper, and the solid wedge indicates both methyls project forward, in front of the plane of the paper. Model 2: Use the solid wedge tool to attach first methyl group to one of two different carbon atoms in the cyclopentane, and use the dashed wedge tool to attach the second. Note the solid wedge indicates that one methyl projects forward, in front of the plane of the paper. The dashed wedge indicates the other methyl extends back, behind the plane of the paper. Click the broom to tidy up the…arrow_forward
- V. Give what is asked. 1. When 2 carbon atoms form a double bond, how many pairs of e- will be shared between them? 2. An alkane has 5 carbon atoms. How many hydrogen atoms will it have? 3. An alkene has 3 carbon atoms. How many hydrogen atoms will it have? 4. An alkyne has 4 carbon atoms. How many hydrogen atoms will it have? 5. Show the Lewis structure for the stable molecule CH3.arrow_forwardConvert each condensed formula to a complete structure with lone pairs on heteroatoms. a. CH 3(CH 2) 8CH 3 c. CH 3CCl 3 e. (CH 3) 2CHCH 2NH 2 b. CH 3(CH 2) 4OH d. CH 3(CH 2) 4CH(CH 3) 2arrow_forwardConvert each molecule to a skeletal structure. a. (CH3)2CHCH2CH2CH(CH3)2 b. CH3CH(Cl)CH(OH)CH3 c.CH3(CH2)2C(CH3)2CH(CH3)CH(CH3)CH(Br)CH3arrow_forward
- Convert the following condensed formulas into skeletal structures. a. CH3CONHCH3 b. CH3COCH2Br c. (CH3)COH d. CH3COCl e. CH3COCH2CO2H f. HO2CCH(OH)CO2Harrow_forwardIdentify the functional groups in each molecule. . CH₂ CH,CH,CO2 CH;C=H Darvon (analgesic) CH₂N(CH3)2 b. HO CH₂ - တစ CH₂ COOH penicillin G (an antibiotic) ibuprofen (analgesic) .arrow_forwardPredict the indicated bond angles in each compound. H a. CH,cc CI b. CH2=CCI c. CH3 C-arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





