
Concept explainers
Interpretation:
A synthetic route of given compounds have to be proposed.
Concept Introduction:
Anti-Markovnikov’s rule: An unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the less substitution position of carbon-carbon double bond and gives
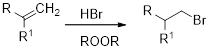
Markovnikov’s rule: An unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the more substitution position of carbon-carbon double bond which provides alkyl halides.

Lindlar reduction: The
Elimination Reaction: It is just reverse reaction of addition reaction where substituent from the given molecule is removed via E1 (the reaction depends only on the substrate involved in the reaction) or E2 (the reaction depends on both of the substituents in the reaction) mechanism.
9-BBN (9-Borabicyclo[3.3.1]nonane) or diborane is used for the hydroboration of alkene. Boron addition to the double bond and subsequent oxidation of the newly formed borane yields anti-Markovnikov alcohols.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





