
A refrigerator operating on the vapor-compression refrigeration cycle using refrigerant-134a as the refrigerant is considered. The temperatures of the cooled space and the ambient air are at 10°F and 80°F, respectively. R-134a enters the compressor at 20 psia as a saturated vapor and leaves at 140 psia and 160°F. The refrigerant leaves the condenser as a saturated liquid. The rate of cooling provided by the system is 45,000 Btu/h. Determine (a) the mass flow rate of R-134a and the COP, (b) the exergy destruction in each component of the cycle and the second-law efficiency of the compressor, and (c) the second-law efficiency of the cycle and the total exergy destruction in the cycle.
(a)
The mass flow rate of R-134a and the COP.
Answer to Problem 30P
The mass flow rate of R-134a and the COP is
Explanation of Solution
Show the T-s diagram for vapor-compression refrigeration cycle as in Figure (1).
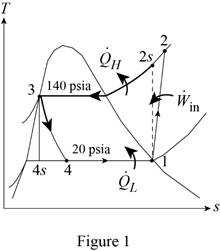
From Figure (1), write the specific enthalpy at state 3 is equal to state 4 due to throttling process.
Here, specific enthalpy at state 3 and 4 is
Express the work input.
Here, specific enthalpy at state 2 and 1 is
Express heat supplied to the cooled space.
Express the heat removed from the cooled space.
Express quality at state 4.
Here, specific enthalpy at saturated liquid and evaporation and
Express specific entropy at state 4.
Here, specific entropy at saturated liquid and evaporation and
Express mass flow rate of R-134a.
Here, rate of heat lost is
Express the COP of the cycle.
Conclusion:
Refer Table A-12E, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to initial pressure
Here, specific entropy at state 1 is
Refer Table A-13E, “superheated refrigerant-134a”, and write the properties corresponding to pressure at state 2
Here, specific entropy at state 2 is
Refer Table A-12E, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to pressure at state 3
Here, specific entropy at state 3 is
As specific enthalpy at state 3 is equal to specific enthalpy at state 4,
Refer Table A-12E, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to pressure at state 4
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Hence, the mass flow rate of R-134a and the COP is
(b)
The exergy destruction in each component of the cycle and the second-law efficiency of the compressor.
Answer to Problem 30P
The exergy destruction in compressor is
Explanation of Solution
For compressor:
Express the exergy destruction in compressor.
Here, surrounding temperature is
For condenser:
Express the exergy destruction in condenser.
Here, entropy generation during process 2-3 is
For expansion valve:
For evaporator:
Express the exergy destruction in evaporator.
Here, entropy generation during process 4-1 is
Express the power input of the compressor.
Express second law efficiency of the compressor.
Conclusion:
Perform unit conversion of surrounding temperature from
Perform unit conversion of high temperature medium from
Perform unit conversion of low temperature medium from
Substitute
Hence, the exergy destruction in compressor is
Substitute
Hence, the exergy destruction in condenser is
Substitute
Hence, the exergy destruction in expansion valve is
Substitute
Hence, the exergy destruction in evaporator is
Substitute
Substitute
Hence, the second-law efficiency of the compressor is
(c)
The second-law efficiency of the cycle and the total exergy destruction in the cycle.
Answer to Problem 30P
The second-law efficiency of the cycle is
Explanation of Solution
Express the exergy of the heat transferred from the low temperature medium.
Determine the second law efficiency of the cycle.
Express the total exergy destruction in the cycle.
Conclusion:
Substitute
Substitute
Hence, the second-law efficiency of the cycle is
Substitute
Hence, the total exergy destruction in the cycle is
Want to see more full solutions like this?
Chapter 11 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- The operating condition for the single compressor in a household refrigerator is the lowest box temperature, which is typically A. 0F B. -20F C. 20F D. 40Farrow_forwardRefrigerators currently being manufactured in the United States are using______as their refrigerant.arrow_forwardCondensers in these refrigerators are all_______cooled.arrow_forward
- An ideal vapor-compression refrigeration cycle uses R-134a as the refrigerant. The refrigerant enters the evaporator at 160 kpa with a quality of 25% and leaves the compressor at 65 °C. If the compressor consumes 800W of power, determine (a) the mass flow rate of the refrigerant, (b) the condenser pressure, and (c) the COP of the refrigeratorarrow_forwardAn ideal refrigerant-134a vapor-compression refrigeration cycle operates between 0.14 MPa and 0.7 MPa. The mass flow rate of the refrigerant is 0.15 kg/s. Determine the rate of heat removal from the refrigerated space, kW.arrow_forwardA vapor-compression system produces 20 tons of refrigeration using R12 as a refrigerant while operating between a condenser temperature of 41.6°c and an evaporator temperature of – 25°c. The superheat at the compression suction and the sub-cooling at the condenser outlet are 10°K. The pressure drop are 10kPa across the evaporator and the condenser. Determine the refrigerating effect in kJ per kilogram, the circulating rate of R12 in kilograms per second, the power required, the COP, the heat rejected in kW and the volume flow rate of refrigerant at compressor inlet conditions. 10° K subcooling 4 Tm= 41.6° C h= C s =C Tevap =-25° C 10° K superheatarrow_forward
- A refrigerator uses refrigerant-134a as the working fluid and operates on an ideal vapor compression refrigeration cycle between 0.18 and 0.7 MPa. The mass flow rate of the refrigerant is 72.4 g/s. Determine the power input to the compressor (in kW). Do not answer in image formatarrow_forwardConsider an actual vapor-compression refrigeration cycle. R-134a enters the compressor as superheated vapor at 0.18 MPa and -10°C at a rate of 0.055 kg/s, and it leaves at 1.2 MPa and 60°C. The refrigerant is cooled in the condenser to 42°C and 1.15 MPa, and it is throttled to 0.22 MPa. Determine the rate of heat addition to the evaporator, in kW.arrow_forwardA refrigerator uses refrigerant 134a as the working fluid and operates on an ideal vapor-compression refrigeration cycle between 0.18 Mpa and 1.0 Mpa. The mass flow rate of the refrigerant is 0.07 kg/s. Determine the COP of the refrigerator.arrow_forward
- A refrigerated room is kept at -27°C by a vapor-compression cycle with R-134a as the refrigerant. Heat is rejected to cooling water that enters the condenser at 16°C at a rate of 0.22 kg/s and leaves at 23 °C. The refrigerant enters the condenser at 1.2 MPa and 65 °C and leaves at 42°C. The inlet state of the compressor is 60 kPa and -34 °C and the compressor is estimated to gain a net heat of 150 W from the surroundings. Determine (a) the quality of the refrigerant at the evaporator inlet, (b) the refrigeration load, (c) the COP of the refrigerator, and (d) the theoretical maximum refrigeration load for the same power input to the compressor.arrow_forwardIn a vapor-compression refrigeration cycle, ammonia exits the evaporator as saturated vapor at -22°C. The refrigerant enters the condenser at 16 bar and 190°C, and saturated liquid exits at 16 bar. There is no significant heat transfer between the compressor and its surroundings, and the refrigerant passes through the evaporator with a negligible change in pressure.If the refrigerating capacity is 50 kW, determine:(a) the mass flow rate of the refrigerant, in kg/s.(b) the power input to the compressor, in kW.(c) the coefficient of performance.(d) the isentropic compressor efficiency, in percent.(e) the rate of entropy production, in kW/K, for the compressor.arrow_forwardA vapor-compression refrigeration system circulates refrigerant 134a at a rate of 0.15 kg/s. The refrigerant enters the compressor at -10 degrees Celcius and 100 kPa, and exits the compressor at 800 kPa. The isentropic efficiency of the compressor is 76%. Pressure drop through the condenser and evaporator are negligible. The refrigerant exits the condenser at 30 degrees Celcius and 800 kPa. Ignoring the heat transfer between the compressor and its surroundings, determine: The rate at which heat energy is removed from the refrigerated space in kW. The coefficient of perfromance.arrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
