
(a)
Interpretation:
The moment of inertia of a linear molecule of the form ABC has to be derived.
Concept Introduction:
Moment of inertia:
The moment of inertia of a molecule is the mass of each atom multiplied by the square of its perpendicular distance from the axis of rotation.
(a)
Explanation of Solution
Consider a triatomic linear molecule of the form ABC. Let,
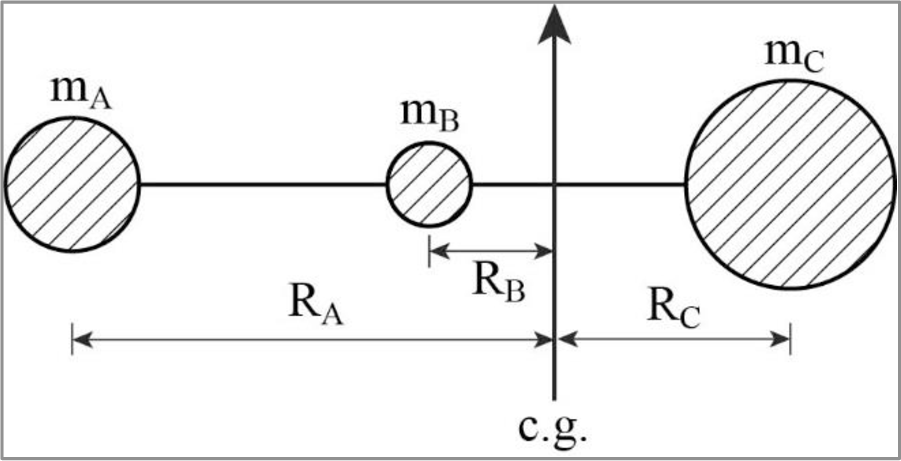
Figure.1
Now,
Moment around the center of gravity,
Then,
Now substitute the value of equation 3 in equation 2.
Now substituting the equation 4 in equation 1,
Now substituting the value of
It can be rearranged as
This is the expression for the moment of inertia of a triatomic linear molecule of the form ABC.
(b)
Interpretation:
The rotational constants and moment of inertia of
Concept Introduction:
The gap between two adjacent spectral lines is
(b)
Answer to Problem 11.5P
The rotational constants of
Explanation of Solution
Given data:

Figure.2
Calculation of rotational constants:
For
The gap between the two spectral lines,
Now
For
The gap between the two spectral lines,
Now
Therefore, the rotational constants for
Calculation of moment of inertia:
For
As
So for
So for
Therefore, the moment of inertia of
(c)
Interpretation:
The
Concept Introduction:
The bond length of a given molecule can be found out from its moment of inertia as moment of inertia is the product of mass and bond length.
(c)
Answer to Problem 11.5P
The
Explanation of Solution
The moment of inertia of
It can be arranged further,
Now
Where,
After putting the value of moment of inertia into the above equation,
After solving this complex equations, the outcome is
Want to see more full solutions like this?
Chapter 11 Solutions
Elements Of Physical Chemistry
- Show work with explanation needed. don't give Ai generated solutionarrow_forwardShow work with explanation needed. Don't give Ai generated solutionarrow_forward7. Calculate the following for a 1.50 M Ca(OH)2 solution. a. The concentration of hydroxide, [OH-] b. The concentration of hydronium, [H3O+] c. The pOH d. The pHarrow_forward
- A first order reaction is 46.0% complete at the end of 59.0 minutes. What is the value of k? What is the half-life for this reaction? HOW DO WE GET THERE? The integrated rate law will be used to determine the value of k. In [A] [A]。 = = -kt What is the value of [A] [A]。 when the reaction is 46.0% complete?arrow_forward3. Provide the missing compounds or reagents. 1. H,NNH КОН 4 EN MN. 1. HBUCK = 8 хно Panely prowseful kanti-chuprccant fad, winddively, can lead to the crading of deduc din-willed, tica, The that chemooices in redimi Грин. " like (for alongan Ridovi MN نيا . 2. Cl -BuO 1. NUH 2.A A -BuOK THE CF,00,H Ex 5)arrow_forward2. Write a complete mechanism for the reaction shown below. NaOCH LOCH₁ O₂N NO2 CH₂OH, 20 °C O₂N NO2arrow_forward
- 4. Propose a synthesis of the target molecules from the respective starting materials. a) b) LUCH C Br OHarrow_forwardThe following mechanism for the gas phase reaction of H2 and ICI that is consistent with the observed rate law is: step 1 step 2 slow: H2(g) +ICI(g) → HCl(g) + HI(g) fast: ICI(g) + HI(g) → HCl(g) + |2(g) (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. + → + (2) Which species acts as a catalyst? Enter formula. If none, leave box blank: (3) Which species acts as a reaction intermediate? Enter formula. If none, leave box blank: (4) Complete the rate law for the overall reaction that is consistent with this mechanism. (Use the form k[A][B]"..., where '1' is understood (so don't write it) for m, n etc.) Rate =arrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forward
- 1. For each of the following statements, indicate whether they are true of false. ⚫ the terms primary, secondary and tertiary have different meanings when applied to amines than they do when applied to alcohols. • a tertiary amine is one that is bonded to a tertiary carbon atom (one with three C atoms bonded to it). • simple five-membered heteroaromatic compounds (e.g. pyrrole) are typically more electron rich than benzene. ⚫ simple six-membered heteroaromatic compounds (e.g. pyridine) are typically more electron rich than benzene. • pyrrole is very weakly basic because protonation anywhere on the ring disrupts the aromaticity. • thiophene is more reactive than benzene toward electrophilic aromatic substitution. • pyridine is more reactive than nitrobenzene toward electrophilic aromatic substitution. • the lone pair on the nitrogen atom of pyridine is part of the pi system.arrow_forwardThe following reactions are NOT ordered in the way in which they occur. Reaction 1 PhO-OPh Reaction 2 Ph-O -CH₂ heat 2 *OPh Pho -CH2 Reaction 3 Ph-O ⚫OPh + -CH₂ Reaction 4 Pho Pho + H₂C OPh + CHOPh H₂C -CH₂ Reactions 1 and 3 Reaction 2 O Reaction 3 ○ Reactions 3 and 4 ○ Reactions 1 and 2 Reaction 4 ○ Reaction 1arrow_forwardSelect all possible products from the following reaction: NaOH H₂O a) b) ОН HO O HO HO e) ОН f) O HO g) h) + OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





