
(a)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(a)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
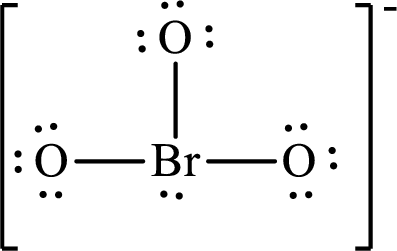
Boron forms three single bonds with three oxygen atoms and one lone pair is present on it so four hybrid orbitals are required and therefore the hybridization of boron in
The presence of lone pair on the central atom results in the deviation of bond angles from that of the ideal ones.
(b)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(b)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
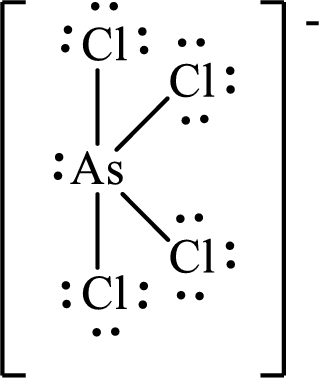
Arsenic forms four single bonds with four chlorine atoms and one lone pair is present on it so five hybrid orbitals are required and therefore the hybridization of arsenic in
The presence of lone pair on the central atom results in the deviation of bond angles from that of the ideal ones.
(c)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(c)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
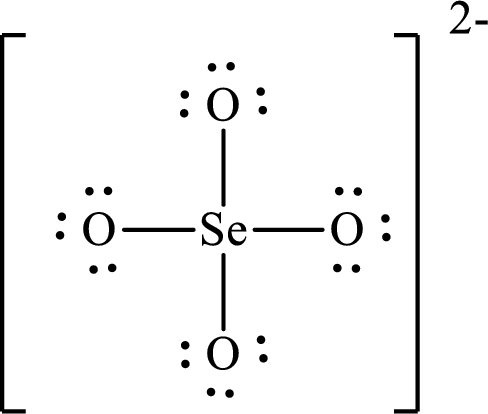
Selenium forms four single bonds with four oxygen atoms so four hybrid orbitals are required and therefore the hybridization of selenium in
The shape of the molecule is determined by the hybridization only if no lone pair is present on the central atom.
(d)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(d)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
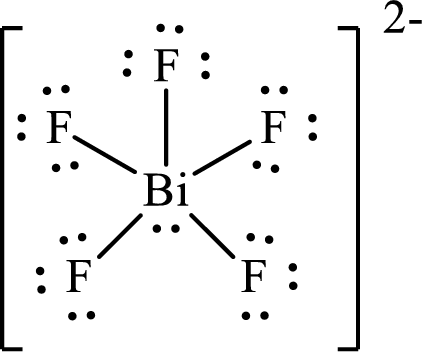
Bismuth forms five single bonds with five fluorine atoms and one lone pair is present on it so six hybrid orbitals are required and therefore the hybridization of bismuth in
The presence of lone pair on the central atom results in the deviation of bond angles from that of the ideal ones.
(e)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(e)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
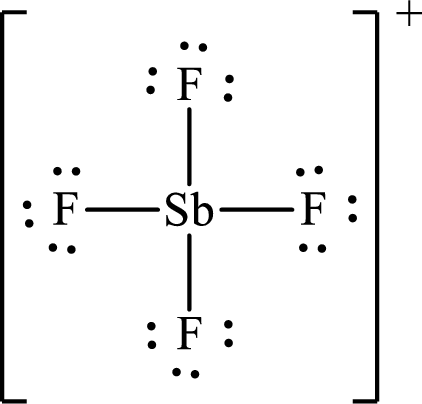
Antimony forms four single bonds with four fluorine atoms so four hybrid orbitals are required and therefore the hybridization of antimony in
The shape of the molecule is determined by the hybridization only if no lone pair is present on the central atom.
(f)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(f)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
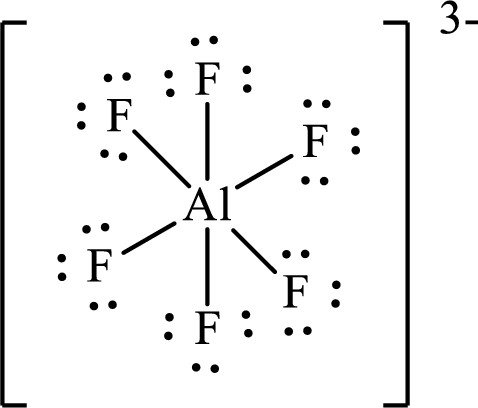
Aluminium forms six single bonds with six fluorine atoms so six hybrid orbitals are required and therefore the hybridization of aluminium in
The shape of the molecule is determined by the hybridization only if no lone pair is present on the central atom.
(g)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(g)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
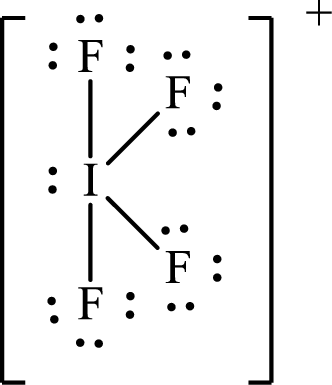
Iodine forms four single bonds with four fluorine atoms and one lone pair is present on it so five hybrid orbitals are required and therefore the hybridization of iodine in
The presence of lone pair on the central atom results in the deviation of bond angles from that of the ideal ones.
Want to see more full solutions like this?
Chapter 11 Solutions
CHEMISTRY >CUSTOM<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





