
Interpretation:
The
Concept introduction:
There are primarily four types of acid-base titrations –
- Strong base vs strong acid
- Strong base vs weak acid
- Weak base vs strong acid
- Weak base vs weak acid
Equivalence point ensures the titration reaction becomes completed and at this point the number of moles of titrant and the number of moles of analyte becomes equal.
Explanation of Solution
Piperazine is a weak base and
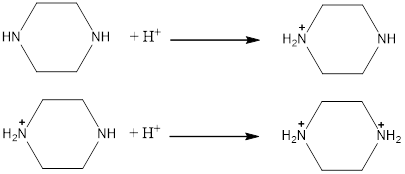
Piperazine has two N-atoms and each N-atom has a pair of non-bonding electrons available for bonding which makes the molecule basic. The protons from
Given data:
When volume of acid added
Then,
The base has two
Solving for ‘x’,
Therefore
Therefore
Therefore
Therefore
Solving the above equation for

Therefore
Therefore
Therefore
All of B is converted to
Therefore
Then,
Dissociation constant Ka is,
Solving for ‘x’,
Therefore
Therefore
Therefore
For each volume of acid added corresponding
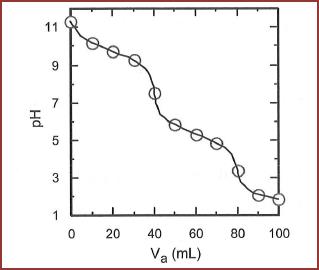
Figure 1
The
Want to see more full solutions like this?
Chapter 11 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





