
(a)
Interpretation:
Concentration of each species in below diagram has to be calculated.
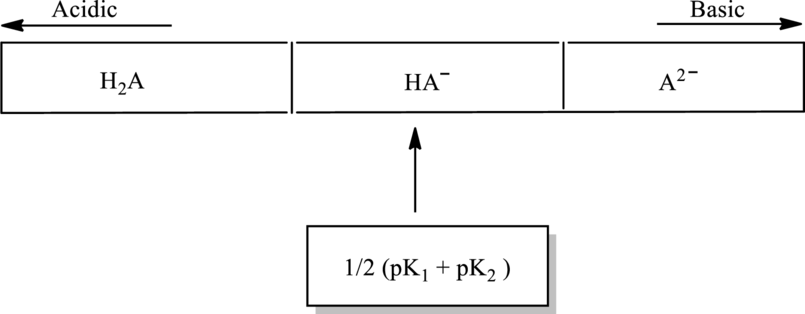
Concept Introduction:
The equation for buffer is described by Henderson-Hasselbach equation and it is a rearranged for of equilibrium constant,
Formula to calculate
Here,
(a)
Explanation of Solution
In accordance to Henderson Hasselbach equation
Substitute
At
In accordance to Henderson Hasselbach equation
In accordance to Henderson Hasselbach equation
The expression for final
Substitute
Substitute
At
In accordance to Henderson Hasselbach equation
Substitute
At
(b)
Interpretation:
Analogous diagrams for monoprotic and triprotic systems have to be drawn.
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
In diagram of monoprotic system if
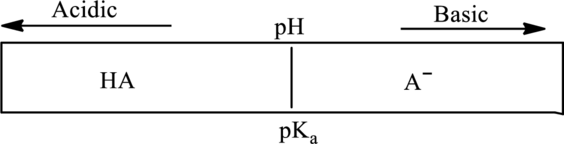
In accordance to Henderson Hasselbach equation
Substitute
At
In diagram of triprotic system diagram consist of
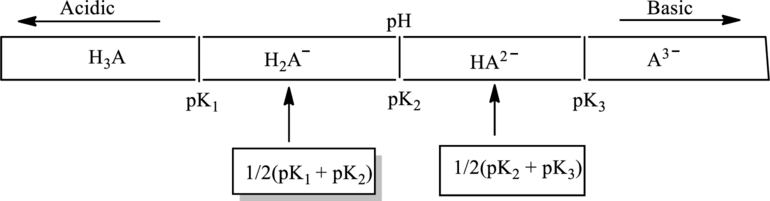
In accordance to Henderson Hasselbach equation
Substitute
At
In accordance to Henderson Hasselbach equation
In accordance to Henderson Hasselbach equation
The expression for final
Substitute
Substitute
At
In accordance to Henderson Hasselbach equation
Substitute
At
In accordance to Henderson Hasselbach equation
In accordance to Henderson Hasselbach equation
The expression for final
Substitute
Substitute
At
In accordance to Henderson Hasselbach equation
Substitute
At
Want to see more full solutions like this?
Chapter 11 Solutions
Exploring Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





