
Concept explainers
a)
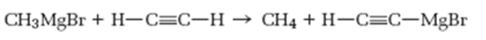
Interpretation:
As a base how much strong Grignard reagent is to be stated. Further based on the pKa values of
Concept introduction:
Stronger acids have low pKa values. A strong acid will yield a weak conjugate base. Similarly Weak acids will give strong conjugate bases. A strong base will readily remove a proton from a weak acid.
To state:
How much strong Grignard reagent is and to predict based on the pKa values of alkanes and alkynes whether the reaction shown will occur as written.
b)
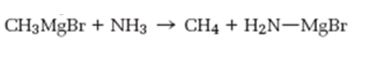
Interpretation:
As a base how much strong Grignard reagent is to be stated. Further Based on the pKa values of alkanes and ammonia to predict whether the reaction shown will occur as written.
Concept introduction:
Stronger acids have low pKa values. A strong acid will yield a weak conjugate base. Similarly weak acids will give strong conjugate bases. A strong base will readily remove a proton from a weak acid.
To state:
How much strong Grignard reagent is and to predict based on the pKa values of alkanes and ammonia whether the reaction shown will occur as written.
Trending nowThis is a popular solution!

Chapter 10 Solutions
Organic Chemistry
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


