
Concept explainers
Interpretation:
The radicals, in order of theirs decreasing stability, are to be listed.
Concept introduction:
A molecule that contains at least one unpaired electron is known as a radical.
The relative stability of radicals is the same as that of carbocations.
The stability of radicals is as follows:
Answer to Problem 1PP
Solution:
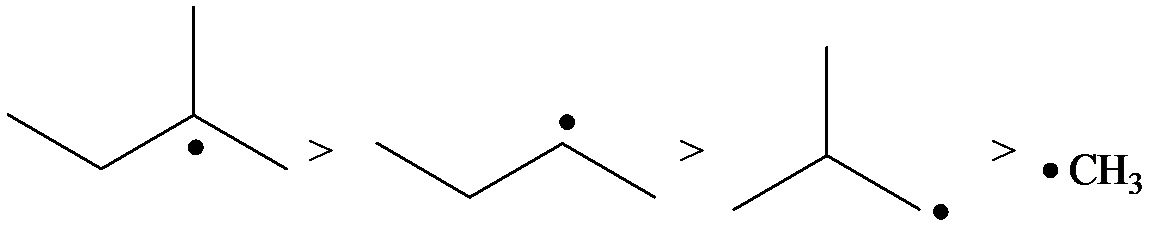
Explanation of Solution

The radical is a methyl radical. So, it is the least stable.
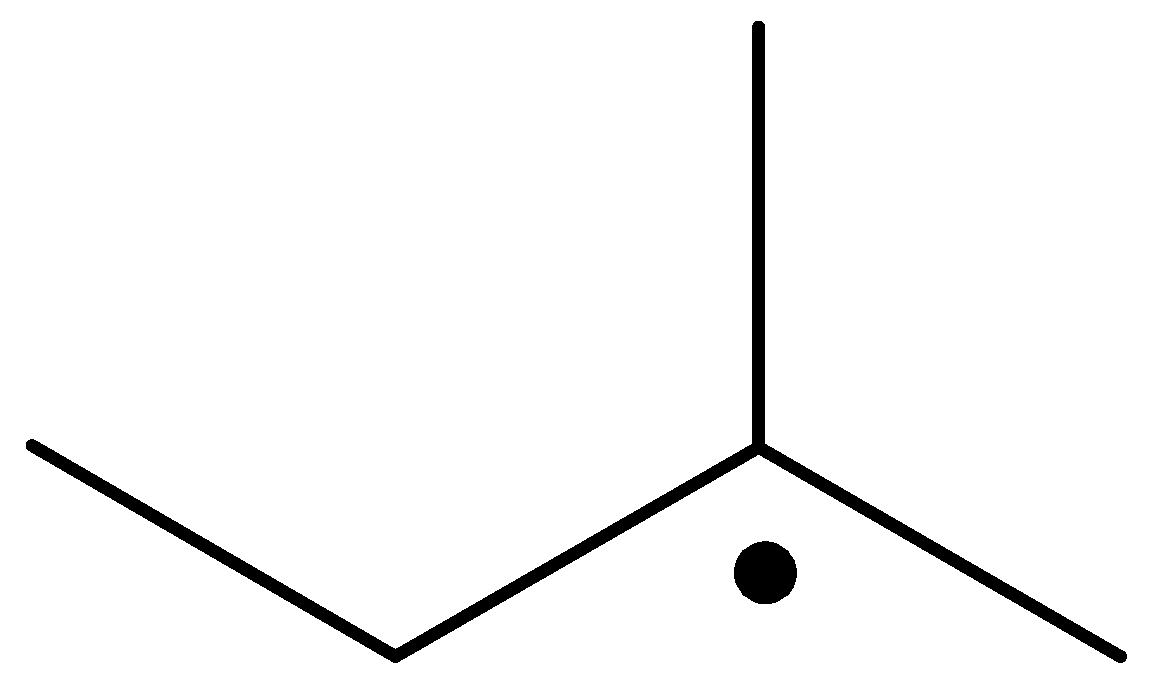
The radical is given as follows:
The carbon containing the unpaired electron is primary. Thus, the radical is a primary radical.
The radical is given as follows:
The carbon containing the unpaired electron is tertiary. Thus, the radical is a tertiary radical.
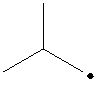
The radical is given as follows:
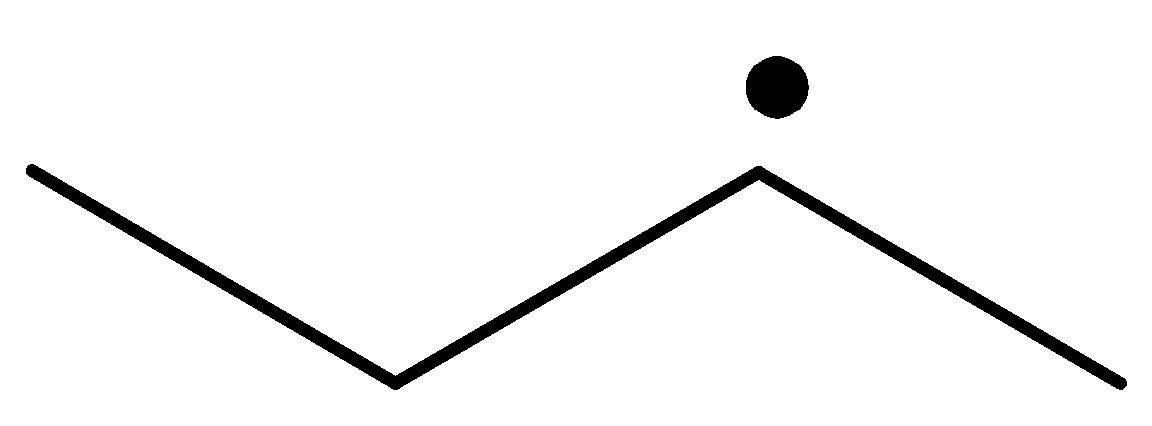
The carbon containing the unpaired electron is secondary. Thus, the radical is a secondary radical.
Therefore, the tertiary radical is the most stable and the methyl radical is the least stable.
Hence, the decreasing order of the stability of the radical is as follows:
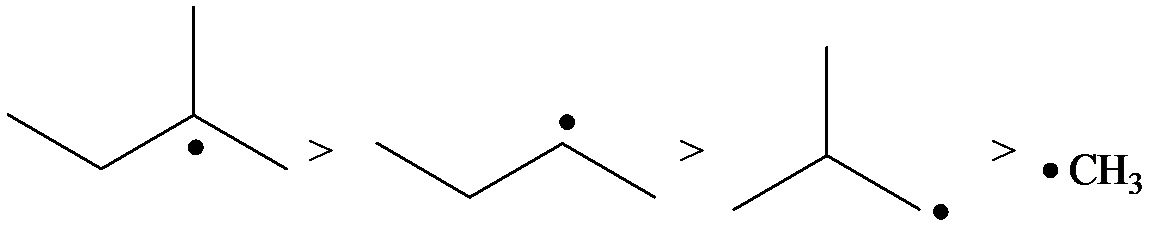
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
- • PRACTICE PROBLEM 8.24 A, B, and C are alkynes. Elucidate their structures and that of D using the following reaction roadmap. H2, Pt H,, Pt A (C3H14) (C3H14) IR: 3300 cm (1) O3 (2) HOẠC HO, H2, Pt (C3H12) (C3H16) (1) O3 (2) HOAC hol bian vd beeollot Но. ОН AOHarrow_forward10.36 A very large number of Diels-Alder reactions are recorded in the chemical literature, man O your knowl of which involve relatively complicated dienes, dienophiles, or both. On the basis of edge of Diels-Alder reactions, predict the constitution of the Diels-Alder adduct that you would expect to be formed from the following combinations of dienes and dienophiles: Also (b) 7 O + CH₂O₂CC=CCO₂CH, -carbocation/radical structure, stability and resonancearrow_forwardArrange the following in increasing order of stability:arrow_forward
- For centuries, Chinese herbal medicine has used extracts of Ephedra sinica to treat asthma. Ephedra as an"herbal supplement" has been implicated in the deaths of several athletes and has recently been banned as a dietary supplement. Phytochemical investigation of this plant resulted in isolation of ephedrine, a very potent dilator of the air passages of the lungs. Ephedrine also has profound effects on the cardiovascular system. The natu- rally occurring stereoisomer is levorotatory and has the following structure. Assign an Ror S configuration to each chiral center. H NHCH3 Ephedra sinica, the source of ephedrine, a potent bronchodilator. HO H CH3 Ephedrine [a -41arrow_forwardCompound A, C₂H16 reacts with 1 molar equivalent(s) of hydrogen on catalytic hydrogenation. A undergoes reaction with ozone, followed by Zn treatment, to give: O CH3 O || CH3CCH₂CCH₂CCH3 CH3 Compound A Propose a structure for A. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forward1) Azulene, bicyclo[5.3.0] decapentaene, is an beautiful blue colored hydrocarbon with a large dipole moment (for a hydrocarbon). Draw resonance structures that could explain the large dipole moment. Azulenearrow_forward
- Compound X, C,4H12Br2, is optically inactive. On treatment with strong base, X gives hydrocarbon Y, C14H10: Compound Y absorbs 2 equivalents of hydrogen when reduced over a palladium catalyst to give z (C14H14) and reacts with ozone to give one product, benzoic acid (C,Hg02). Draw the structure of compound Z. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • Ignore alkene stereochemistry. • If more than one structure fits the description, draw them all. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu. ChemDoodlearrow_forwardRank the following set of compounds in order of increasing bond λmax.arrow_forwardProvide the bond line structure of a compound that is able to undergo Allylic lone pair and allylic carbocation resonance. Provide all resonance structures and appropriate arrows. Indicate which resonance state is most stable and why.arrow_forward
- (b) Study the two reactions below and determine if they can form a new product or if no reaction occurs. If a reaction can occur provide the structure of the product. For each reaction draw the conformations, provide the mechanisms and discuss the stereoelectronics to explain your answer. Option 1: E OH LL Br Me Br KO'Bu KO'Buarrow_forwardRank by the stability of the alkene isomers. The most stable isomer is 1, while the least stable isomer is 5. (A) (B) (C) (D) (E)arrow_forwardConsider the reaction between (1S,3S)‑1‑chloro‑3‑methylcyclopentane and methanethiol in the presence of sodium hydroxide. (a) Draw the organic product and clearly indicate stereochemistry by showing the hydrogen on the chirality centers and using wedge and dash bonds. (b) Then analyze the stereochemistry of the product. racemic chiral achiral (1R, 3S) (1R, 3R) (1S, 3S)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
