
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Complete the following table for aqueous solutions of potassium hydroxide.
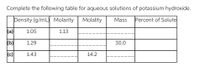
Transcribed Image Text:Complete the following table for aqueous solutions of potassium hydroxide.
Density (g/mL) Molarity
Molality
Mass
Percent of Solute
(a)
1.05
1.13
(b)
1.29
30.0
(c)
1.43
14.2

Transcribed Image Text:ON
DATE:
Copper leaction balanced ch emi cal heaction
A) Copper metal "disrolves"in hitric Acid
B) Hydroxide ion add to the copper (1).
Icu (H,0),72(ag) + 2 OH"-> Cu CoH), (S) + GH, O (!)
) Heating Copper Hydroxide.
Cu (OH)2 (s) -> (40()+ H2 01)
) copper Oxide discolve in a cid
E) Zinc metal lessen the hydrdted copper
zinc metal le sten the hydranium lcns to Hhs
2n (6) + 2HgOt (ag) -> Zn *t (ag) t Hz (g)+2 H20()
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many grams of NaOH are required to prepare 250.0 mL of a 0.900 M sodium hydroxide solution?arrow_forwardBy titration, it is found that 55.5 mL of 0.116 M NaOH(aq) is needed to neutralize 25.0 mL of HCl(aq). Calculate the concentration of the HCl solution. [HCI] Marrow_forwardBy titration it is found that 35.7 mL of 0.101 M NaOH(aq) is needed to neutralize 25.0 mL of HCl(aq). Calculate the concentration of the HCl solution.arrow_forward
- Calculate the mass of NaClNaCl in a 38 −mL−mL sample of a 1.3 MM NaClNaCl solution.arrow_forwardCalculate the concentration of sulfate (SO4^-2) ions in a 2.5 M aqueous solution of Al2(SO4)3. Report your answer to the correct units.arrow_forwardYou have a 1 M stock solution of Tris-HCl. Calculate how much of it you would need to make 100 ml of a 1 mM solution of Tris-HCl. Be sure to include an unit in your answer.arrow_forward
- write the balanced chemical equation for the reaction between phosphoric acid and iron(II) hydroxide. Calculate the mass of precipitate that can be formed when starting with 15.79 mL of 0.091 M phosphoric acid.arrow_forwardIf 47.1 g of C₂H5OH (MM = 46.07 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of C₂H5OH in the resulting solution?arrow_forwardA 0.249 M sodium hydroxide solution required 25.00 mL to titrate 10.0 mL of an unknown concentration of sulfuric acid. Calculate the molarity of the sulfuric acid solution.arrow_forward
- It takes 23.98mLs of 0.05M HCl to react completely with 30.00mLs of a barium hydroxide solution. What is the molarity of the barium hydroxide solution?arrow_forwardWhat is the mass of zinc hydroxide dissolved in 1500.0 mL of 0.210 M zinc hydroxide solution?arrow_forwardThe concentration of 149mL of an aqueous solution of KNO2 is 2.25 M. How many grams of KNO2 are used to prepare this solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY