
Interpretation:
The potential energy of two electric dipole moments in the given structure is represented by the given expression for the dipole-dipole interaction energy has to be shown.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Interaction between partial charges:
Explanation of Solution
The expression for the potential energy of two parallel dipole moments is the sum of the interaction between the partial charges and is given below:
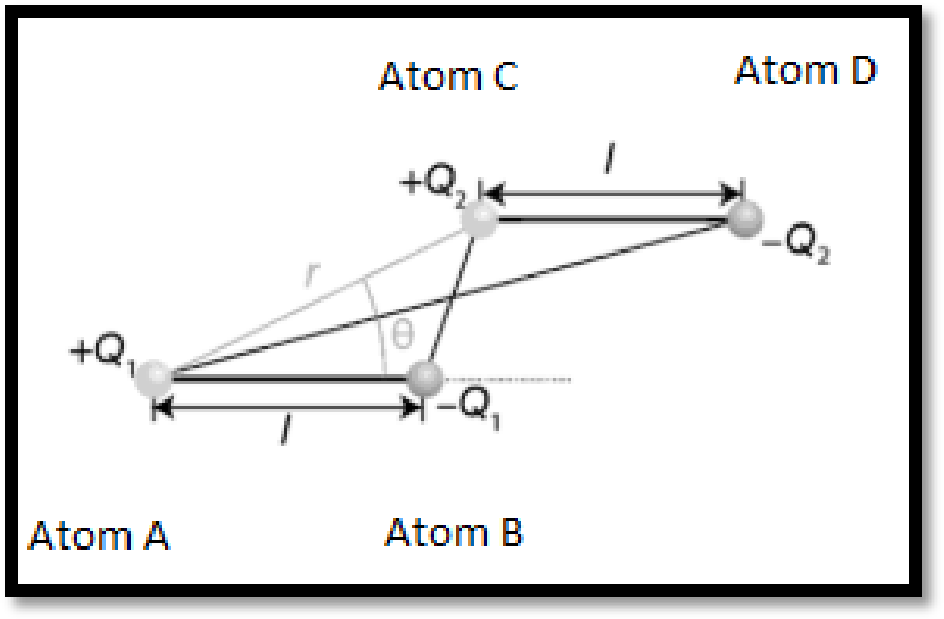
From the above figure, the separation between like charges,
The separation between opposite charges can be given by trigonometry as given below:
Therefore,
The function of the form
Thus,
On substituting,
Want to see more full solutions like this?
Chapter 10 Solutions
Elements Of Physical Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





